[English] 日本語
 Yorodumi
Yorodumi- PDB-2bya: Is radiation damage dependent on the dose-rate used during macrom... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2bya | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Is radiation damage dependent on the dose-rate used during macromolecular crystallography data collection | |||||||||
 Components Components | CATIONIC TRYPSIN | |||||||||
 Keywords Keywords | HYDROLASE / DATA COLLECTION / RADIATION DAMAGE / DOSE-RATE / SYNCHROTRON RADIATION | |||||||||
| Function / homology |  Function and homology information Function and homology informationtrypsin / serpin family protein binding / serine protease inhibitor complex / digestion / endopeptidase activity / serine-type endopeptidase activity / proteolysis / extracellular space / metal ion binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / OTHER / Resolution: 1.3 Å SYNCHROTRON / OTHER / Resolution: 1.3 Å | |||||||||
 Authors Authors | Leiros, H.-K.S. / Timmins, J. / Ravelli, R.B.G. / McSweeney, S.M. | |||||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.D / Year: 2006 Journal: Acta Crystallogr.,Sect.D / Year: 2006Title: Is Radiation Damage Dependent on the Dose-Rate Used During Macromolecular Crystallography Data Collection? Authors: Leiros, H.-K.S. / Timmins, J. / Ravelli, R.B.G. / Mcsweeney, S.M. | |||||||||
| History |
| |||||||||
| Remark 700 | SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "XB" IN EACH CHAIN ON SHEET RECORDS BELOW ... SHEET DETERMINATION METHOD: DSSP THE SHEETS PRESENTED AS "XB" IN EACH CHAIN ON SHEET RECORDS BELOW IS ACTUALLY AN 6-STRANDED BARREL THIS IS REPRESENTED BY A 7-STRANDED SHEET IN WHICH THE FIRST AND LAST STRANDS ARE IDENTICAL. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2bya.cif.gz 2bya.cif.gz | 122.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2bya.ent.gz pdb2bya.ent.gz | 95.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2bya.json.gz 2bya.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/by/2bya https://data.pdbj.org/pub/pdb/validation_reports/by/2bya ftp://data.pdbj.org/pub/pdb/validation_reports/by/2bya ftp://data.pdbj.org/pub/pdb/validation_reports/by/2bya | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2bxyC  2bxzC  2by0C  2by1C  2by2C  2by3C  2by5C  2by6C  2by7C  2by8C  2by9C C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules X
| #1: Protein | Mass: 25444.717 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|
-Non-polymers , 5 types, 426 molecules 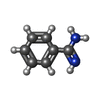








| #2: Chemical | ChemComp-BEN / | ||||
|---|---|---|---|---|---|
| #3: Chemical | ChemComp-GOL / | ||||
| #4: Chemical | | #5: Chemical | ChemComp-CA / | #6: Water | ChemComp-HOH / | |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.73 Å3/Da / Density % sol: 28.42 % |
|---|---|
| Crystal grow | Details: 25% PEG 8000, 0.2 M AMMONIUM SULPHATE AND 0.1 M TRIS-HCL PH 8.0 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID29 / Wavelength: 0.984 / Beamline: ID29 / Wavelength: 0.984 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.984 Å / Relative weight: 1 |
| Reflection | Resolution: 1.3→20 Å / Num. obs: 52141 / % possible obs: 98.8 % / Observed criterion σ(I): 0 / Redundancy: 3.8 % / Biso Wilson estimate: 8.25 Å2 / Rmerge(I) obs: 0.06 / Net I/σ(I): 0 |
| Reflection shell | Resolution: 1.3→1.37 Å / Redundancy: 2.8 % / Rmerge(I) obs: 0.23 / Mean I/σ(I) obs: 3.6 / % possible all: 93 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure: OTHER / Resolution: 1.3→19.87 Å / Cor.coef. Fo:Fc: 0.982 / Cor.coef. Fo:Fc free: 0.971 / SU B: 1.175 / SU ML: 0.022 / Cross valid method: THROUGHOUT / ESU R: 0.038 / ESU R Free: 0.039 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 7.96 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.3→19.87 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




















































































































































































































































 PDBj
PDBj





