+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1xuh | ||||||
|---|---|---|---|---|---|---|---|
| Title | TRYPSIN-KETO-BABIM-CO+2, PH 8.2 | ||||||
 Components Components | TRYPSIN | ||||||
 Keywords Keywords | SERINE PROTEASE / COMPLEX / TRYPSIN-COBALT-SMALL MOLECULE LIGAND / DESIGNED SMALL MOLECULE LIGAND WITH MICROMOLAR AFFINITY | ||||||
| Function / homology |  Function and homology information Function and homology informationtrypsin / serpin family protein binding / serine protease inhibitor complex / digestion / endopeptidase activity / serine-type endopeptidase activity / proteolysis / extracellular space / metal ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.2 Å X-RAY DIFFRACTION / Resolution: 2.2 Å | ||||||
 Authors Authors | Katz, B.A. / Clark, J.M. / Finer-Moore, J.S. / Jenkins, T.E. / Johnson, C.R. / Rose, M.J. / Luong, C. / Moore, W.R. / Stroud, R.M. | ||||||
 Citation Citation |  Journal: Nature / Year: 1998 Journal: Nature / Year: 1998Title: Design of potent selective zinc-mediated serine protease inhibitors. Authors: Katz, B.A. / Clark, J.M. / Finer-Moore, J.S. / Jenkins, T.E. / Johnson, C.R. / Ross, M.J. / Luong, C. / Moore, W.R. / Stroud, R.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1xuh.cif.gz 1xuh.cif.gz | 73 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1xuh.ent.gz pdb1xuh.ent.gz | 54 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1xuh.json.gz 1xuh.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xu/1xuh https://data.pdbj.org/pub/pdb/validation_reports/xu/1xuh ftp://data.pdbj.org/pub/pdb/validation_reports/xu/1xuh ftp://data.pdbj.org/pub/pdb/validation_reports/xu/1xuh | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1c1nC  1c1oC  1c1pC  1c1qC  1c1rC  1c1tC  1c1uC  1c1vC  1c1wC  1c2dC  1c2eC  1c2fC  1c2gC  1c2hC  1c2iC  1c2jC  1c2kC  1c2lC  1c2mC  1xufC  1xugC  1xuiC  1xujC  1xukC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 23324.287 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|
-Non-polymers , 5 types, 129 molecules 


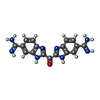





| #2: Chemical | ChemComp-CO / |
|---|---|
| #3: Chemical | ChemComp-CA / |
| #4: Chemical | ChemComp-SO4 / |
| #5: Chemical | ChemComp-BAO / |
| #6: Water | ChemComp-HOH / |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.05 Å3/Da / Density % sol: 14.7 % Description: DATA WITH RSYM > 50 % WERE REJECTED ALONG WITH DATA WHOSE VALUES WERE > 3.5 SIGMA FROM THE MEAN FOR EACH SET OF SYMMETRY EQUIVALENTS |
|---|---|
| Crystal grow | pH: 8.2 Details: SYNTHETIC MOTHER LIQUOR = 75% SATURATED MAGNESIUM SULFATE, 25 % 1.0 M TRIS ADJUSTED TO PH 8.2; SATURATED IN KETO-BABIM, 5.0 MM CO+2. COMPLEX PRODUCED BY SOAKING TRYPSIN-BENZAMIDINE CO-CRYSTAL. |
| Crystal | *PLUS |
| Crystal grow | *PLUS Method: unknown |
-Data collection
| Diffraction | Ambient temp details: ROOM |
|---|---|
| Diffraction source | Wavelength: 1.5418 |
| Detector | Type: SIEMENS-NICOLET X100 / Detector: AREA DETECTOR |
| Radiation | Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Highest resolution: 2.45 Å / Num. obs: 7146 / % possible obs: 76.4 % / Redundancy: 2.1 % / Rmerge(I) obs: 0.128 |
| Reflection shell | Resolution: 2.37→2.47 Å / % possible all: 47.1 |
| Reflection | *PLUS Highest resolution: 2.37 Å / Lowest resolution: 7.5 Å / Num. measured all: 15007 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Highest resolution: 2.2 Å / σ(F): 1 Details: RESIDUES SIMULTANEOUSLY REFINED IN TWO OR MORE CONFORMATIONS ARE: SER88, SER110, LEU1123, GLN135, SER170, SER190, GLN192, SER236, ILE242. BULK SOLVENT WAS REFINED. HIS40 AND HIS91 ARE ...Details: RESIDUES SIMULTANEOUSLY REFINED IN TWO OR MORE CONFORMATIONS ARE: SER88, SER110, LEU1123, GLN135, SER170, SER190, GLN192, SER236, ILE242. BULK SOLVENT WAS REFINED. HIS40 AND HIS91 ARE MONOPROTONATED ON THE EPSILON NITROGEN, HIS57 IS MONOPROTONATED ON THE DELTA NITROGEN.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 2.2 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Xplor file |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Name:  X-PLOR / Classification: refinement X-PLOR / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.37 Å / Lowest resolution: 7.5 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | *PLUS Highest resolution: 2.37 Å / Lowest resolution: 2.47 Å |
 Movie
Movie Controller
Controller























 PDBj
PDBj






