+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1v2v | ||||||
|---|---|---|---|---|---|---|---|
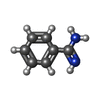
| Title | Benzamidine in complex with bovine trypsin variant X(SSAI)bT.C1 | ||||||
 Components Components | Trypsin | ||||||
 Keywords Keywords | HYDROLASE / SERINE PROTEASE / SERINE PROTEINASE | ||||||
| Function / homology |  Function and homology information Function and homology informationtrypsin / serpin family protein binding / serine protease inhibitor complex / digestion / endopeptidase activity / serine-type endopeptidase activity / proteolysis / extracellular space / metal ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  FOURIER SYNTHESIS / Resolution: 1.8 Å FOURIER SYNTHESIS / Resolution: 1.8 Å | ||||||
 Authors Authors | Rauh, D. / Klebe, G. / Stubbs, M.T. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2004 Journal: J.Mol.Biol. / Year: 2004Title: Understanding protein-ligand interactions: the price of protein flexibility Authors: Rauh, D. / Klebe, G. / Stubbs, M.T. #1:  Journal: J.Mol.Biol. / Year: 2003 Journal: J.Mol.Biol. / Year: 2003Title: ZZ made EZ: influence of inhibitor configuration on enzyme selectivity. Authors: Rauh, D. / Klebe, G. / Sturzebecher, J. / Stubbs, M.T. #2:  Journal: Biol.Chem. / Year: 2002 Journal: Biol.Chem. / Year: 2002Title: Trypsin mutants for structure-based drug design: expression, refolding and crystallisation. Authors: Rauh, D. / Reyda, S. / Klebe, G. / Stubbs, M.T. #3:  Journal: J.Mol.Biol. / Year: 2003 Journal: J.Mol.Biol. / Year: 2003Title: Reconstructing the Binding Site of Factor Xa in Trypsin Reveals Ligand-Induced Structural Plasticity. Authors: Reyda, S. / Sohn, C. / Klebe, G. / Rall, K. / Ullmann, D. / Jakubke, H.D. / Stubbs, M.T. #4:  Journal: Chembiochem / Year: 2002 Journal: Chembiochem / Year: 2002Title: pH-dependent binding modes observed in trypsin crystals: lessons for structure-based drug design. Authors: Stubbs, M.T. / Reyda, S. / Dullweber, F. / Moller, M. / Klebe, G. / Dorsch, D. / Mederski, W.W. / Wurziger, H. #5:  Journal: J.Med.Chem. / Year: 1998 Journal: J.Med.Chem. / Year: 1998Title: Structural and functional analyses of benzamidine-based inhibitors in complex with trypsin: implications for the inhibition of factor Xa, tPA, and urokinase. Authors: Renatus, M. / Bode, W. / Huber, R. / Sturzebecher, J. / Stubbs, M.T. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1v2v.cif.gz 1v2v.cif.gz | 55.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1v2v.ent.gz pdb1v2v.ent.gz | 39.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1v2v.json.gz 1v2v.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/v2/1v2v https://data.pdbj.org/pub/pdb/validation_reports/v2/1v2v ftp://data.pdbj.org/pub/pdb/validation_reports/v2/1v2v ftp://data.pdbj.org/pub/pdb/validation_reports/v2/1v2v | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1v2jC  1v2kC  1v2lC  1v2mC  1v2nC  1v2oC  1v2pC  1v2qC  1v2rC  1v2sC  1v2tC  1v2uC  1v2wC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 23237.211 Da / Num. of mol.: 1 / Mutation: Y172S, P173S, G174A, Q175I Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #2: Chemical | ChemComp-SO4 / |
| #3: Chemical | ChemComp-CA / |
| #4: Chemical | ChemComp-BEN / |
| #5: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.95 Å3/Da / Density % sol: 57.94 % |
|---|---|
| Crystal grow | Temperature: 294 K / Method: vapor diffusion, sitting drop / pH: 6 Details: ammonium sulphate, MES, pH 6, VAPOR DIFFUSION, SITTING DROP, temperature 294K |
-Data collection
| Diffraction | Mean temperature: 287 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU300 / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RU300 / Wavelength: 1.5418 |
| Detector | Type: RIGAKU RAXIS IV / Detector: IMAGE PLATE / Date: Jul 29, 2002 / Details: NI FILTER |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 1.34→46.33 Å / Num. obs: 42089 |
- Processing
Processing
| Software |
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  FOURIER SYNTHESIS / Resolution: 1.8→10 Å / σ(F): 0 / Stereochemistry target values: ENGH & HUBER FOURIER SYNTHESIS / Resolution: 1.8→10 Å / σ(F): 0 / Stereochemistry target values: ENGH & HUBER
| ||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→10 Å
|
 Movie
Movie Controller
Controller






















 PDBj
PDBj