[English] 日本語
 Yorodumi
Yorodumi- PDB-2tgd: LACK OF THE TRANSITION STATE STABILIZATION SITE IS A FACTOR IN TH... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2tgd | ||||||
|---|---|---|---|---|---|---|---|
| Title | LACK OF THE TRANSITION STATE STABILIZATION SITE IS A FACTOR IN THE INACTIVITY OF TRYPSINOGEN, A SERINE PROTEASE ZYMOGEN. STRUCTURE OF DFP INHIBITED BOVINE TRYPSINOGEN AT 2.1 ANGSTROMS RESOLUTION | ||||||
 Components Components | TRYPSINOGEN | ||||||
 Keywords Keywords | HYDROLASE ZYMOGEN (SERINE PROTEINASE) | ||||||
| Function / homology |  Function and homology information Function and homology informationtrypsin / serpin family protein binding / serine protease inhibitor complex / digestion / endopeptidase activity / serine-type endopeptidase activity / proteolysis / extracellular space / metal ion binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.1 Å X-RAY DIFFRACTION / Resolution: 2.1 Å | ||||||
 Authors Authors | Jones, M.O. / Stroud, R.M. | ||||||
 Citation Citation |  Journal: To be Published Journal: To be PublishedTitle: Lack of the Transition State Stabilization Site is a Factor in the Inactivity of Trypsinogen, a Serine Protease Zymogen. Structure of Dfp Inhibited Bovine Trypsinogen at 2.1 Angstroms Resolution Authors: Jones, M.O. / Stroud, R.M. #1:  Journal: Biochemistry / Year: 1977 Journal: Biochemistry / Year: 1977Title: Structure of Bovine Trypsinogen at 1.9 Angstroms Resolution Authors: Kossiakoff, A.A. / Chambers, J.L. / Kay, L.M. / Stroud, R.M. #2:  Journal: Annu.Rev.Biophys.Bioeng. / Year: 1977 Journal: Annu.Rev.Biophys.Bioeng. / Year: 1977Title: Mechanisms of Zymogen Activation Authors: Stroud, R.M. / Kossiakoff, A.A. / Chambers, J.L. | ||||||
| History |
|
- Structure visualization
Structure visualization
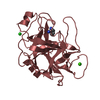
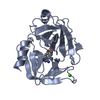
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2tgd.cif.gz 2tgd.cif.gz | 54.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2tgd.ent.gz pdb2tgd.ent.gz | 35.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2tgd.json.gz 2tgd.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tg/2tgd https://data.pdbj.org/pub/pdb/validation_reports/tg/2tgd ftp://data.pdbj.org/pub/pdb/validation_reports/tg/2tgd ftp://data.pdbj.org/pub/pdb/validation_reports/tg/2tgd | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Atom site foot note | 1: SEE REMARK 6. |
- Components
Components
| #1: Protein | Mass: 24012.953 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  |
|---|---|
| #2: Chemical | ChemComp-CA / |
| #3: Chemical | ChemComp-DFP / |
| #4: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2 Å3/Da / Density % sol: 38.42 % |
|---|
- Processing
Processing
| Refinement | Rfactor Rwork: 0.182 / Highest resolution: 2.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement step | Cycle: LAST / Highest resolution: 2.1 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller

















 PDBj
PDBj