| Entry | Database: PDB / ID: 2xgy
|
|---|
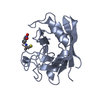
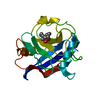
| Title | Complex of Rabbit Endogenous Lentivirus (RELIK)Capsid with Cyclophilin A |
|---|
 Components Components | - PEPTIDYL-PROLYL CIS-TRANS ISOMERASE A
- RELIK CAPSID N-TERMINAL DOMAIN
|
|---|
 Keywords Keywords | VIRAL PROTEIN/ISOMERASE / VIRAL PROTEIN-ISOMERASE COMPLEX / RETROVIRAL CAPSID / ENDOGENOUS |
|---|
| Function / homology |  Function and homology information Function and homology information
negative regulation of protein K48-linked ubiquitination / regulation of apoptotic signaling pathway / cell adhesion molecule production / lipid droplet organization / negative regulation of viral life cycle / heparan sulfate binding / regulation of viral genome replication / virion binding / leukocyte chemotaxis / negative regulation of stress-activated MAPK cascade ...negative regulation of protein K48-linked ubiquitination / regulation of apoptotic signaling pathway / cell adhesion molecule production / lipid droplet organization / negative regulation of viral life cycle / heparan sulfate binding / regulation of viral genome replication / virion binding / leukocyte chemotaxis / negative regulation of stress-activated MAPK cascade / activation of protein kinase B activity / endothelial cell activation / Basigin interactions / protein peptidyl-prolyl isomerization / cyclosporin A binding / Minus-strand DNA synthesis / Plus-strand DNA synthesis / Uncoating of the HIV Virion / Early Phase of HIV Life Cycle / Integration of provirus / APOBEC3G mediated resistance to HIV-1 infection / negative regulation of protein phosphorylation / viral release from host cell / Calcineurin activates NFAT / Binding and entry of HIV virion / negative regulation of oxidative stress-induced intrinsic apoptotic signaling pathway / negative regulation of protein kinase activity / positive regulation of viral genome replication / neutrophil chemotaxis / Gene and protein expression by JAK-STAT signaling after Interleukin-12 stimulation / positive regulation of protein secretion / peptidylprolyl isomerase / peptidyl-prolyl cis-trans isomerase activity / Assembly Of The HIV Virion / : / Budding and maturation of HIV virion / platelet activation / platelet aggregation / integrin binding / positive regulation of protein phosphorylation / neuron differentiation / SARS-CoV-1 activates/modulates innate immune responses / unfolded protein binding / Platelet degranulation / protein folding / cellular response to oxidative stress / secretory granule lumen / vesicle / ficolin-1-rich granule lumen / positive regulation of MAPK cascade / focal adhesion / apoptotic process / Neutrophil degranulation / protein-containing complex / extracellular space / RNA binding / extracellular exosome / extracellular region / membrane / nucleus / cytoplasm / cytosolSimilarity search - Function Human Immunodeficiency Virus Type 1 Capsid Protein / Human Immunodeficiency Virus Type 1 Capsid Protein / Cyclophilin-like / Cyclophilin / Cyclophilin-type peptidyl-prolyl cis-trans isomerase / Cyclophilin-type peptidyl-prolyl cis-trans isomerase, conserved site / Cyclophilin-type peptidyl-prolyl cis-trans isomerase signature. / Cyclophilin-type peptidyl-prolyl cis-trans isomerase domain profile. / Cyclophilin-type peptidyl-prolyl cis-trans isomerase domain / Cyclophilin type peptidyl-prolyl cis-trans isomerase/CLD ...Human Immunodeficiency Virus Type 1 Capsid Protein / Human Immunodeficiency Virus Type 1 Capsid Protein / Cyclophilin-like / Cyclophilin / Cyclophilin-type peptidyl-prolyl cis-trans isomerase / Cyclophilin-type peptidyl-prolyl cis-trans isomerase, conserved site / Cyclophilin-type peptidyl-prolyl cis-trans isomerase signature. / Cyclophilin-type peptidyl-prolyl cis-trans isomerase domain profile. / Cyclophilin-type peptidyl-prolyl cis-trans isomerase domain / Cyclophilin type peptidyl-prolyl cis-trans isomerase/CLD / Cyclophilin-like domain superfamily / Beta Barrel / Orthogonal Bundle / Mainly Beta / Mainly AlphaSimilarity search - Domain/homology |
|---|
| Biological species |   ORYCTOLAGUS CUNICULUS (rabbit) ORYCTOLAGUS CUNICULUS (rabbit)
 HOMO SAPIENS (human) HOMO SAPIENS (human) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å |
|---|
 Authors Authors | Goldstone, D.C. / Robertson, L.E. / Haire, L.F. / Stoye, J.P. / Taylor, I.A. |
|---|
 Citation Citation |  Journal: Cell Host Microbe / Year: 2010 Journal: Cell Host Microbe / Year: 2010
Title: Structural and Functional Analysis of Prehistoric Lentiviruses Uncovers an Ancient Molecular Interface.
Authors: Goldstone, D.C. / Yap, M.W. / Robertson, L.E. / Haire, L.F. / Taylor, W.R. / Katzourakis, A. / Stoye, J.P. / Taylor, I.A. |
|---|
| History | | Deposition | Jun 8, 2010 | Deposition site: PDBE / Processing site: PDBE |
|---|
| Revision 1.0 | Sep 22, 2010 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | May 8, 2011 | Group: Version format compliance |
|---|
| Revision 1.2 | Jul 13, 2011 | Group: Version format compliance |
|---|
| Revision 1.3 | May 8, 2024 | Group: Data collection / Database references ...Data collection / Database references / Derived calculations / Other
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / pdbx_database_status / struct_site
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession ..._database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_database_status.status_code_sf / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 HOMO SAPIENS (human)
HOMO SAPIENS (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å
MOLECULAR REPLACEMENT / Resolution: 1.8 Å  Authors
Authors Citation
Citation Journal: Cell Host Microbe / Year: 2010
Journal: Cell Host Microbe / Year: 2010 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 2xgy.cif.gz
2xgy.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb2xgy.ent.gz
pdb2xgy.ent.gz PDB format
PDB format 2xgy.json.gz
2xgy.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/xg/2xgy
https://data.pdbj.org/pub/pdb/validation_reports/xg/2xgy ftp://data.pdbj.org/pub/pdb/validation_reports/xg/2xgy
ftp://data.pdbj.org/pub/pdb/validation_reports/xg/2xgy Links
Links Assembly
Assembly
 Components
Components

 HOMO SAPIENS (human) / Plasmid: PGEX-6P1 / Production host:
HOMO SAPIENS (human) / Plasmid: PGEX-6P1 / Production host: 
 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation ROTATING ANODE / Wavelength: 1.5418
ROTATING ANODE / Wavelength: 1.5418  Processing
Processing MOLECULAR REPLACEMENT / Resolution: 1.8→24.482 Å / SU ML: 0.31 / σ(F): 1.34 / Phase error: 17.24 / Stereochemistry target values: ML
MOLECULAR REPLACEMENT / Resolution: 1.8→24.482 Å / SU ML: 0.31 / σ(F): 1.34 / Phase error: 17.24 / Stereochemistry target values: ML Movie
Movie Controller
Controller






























































 PDBj
PDBj













