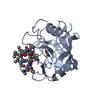
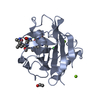
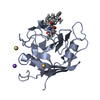
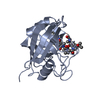
登録情報 データベース : PDB / ID : 1cwmタイトル HUMAN CYCLOPHILIN A COMPLEXED WITH 4 MEILE CYCLOSPORIN CYCLOSPORIN A PEPTIDYL-PROLYL CIS-TRANS ISOMERASE A キーワード / / / / / 機能・相同性 分子機能 ドメイン・相同性 構成要素
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / 生物種 HOMO SAPIENS (ヒト)TOLYPOCLADIUM INFLATUM (菌類)手法 / / 解像度 : 2 Å データ登録者 Mikol, V. / Kallen, J. / Taylor, P. / Walkinshaw, M.D. ジャーナル : J.Mol.Biol. / 年 : 1998タイトル : X-Ray Structures and Analysis of 11 Cyclosporin Derivatives Complexed with Cyclophilin A.著者 : Kallen, J. / Mikol, V. / Taylor, P. / Walkinshaw, M.D. 履歴 登録 1998年5月26日 処理サイト 改定 1.0 1998年7月15日 Provider / タイプ 改定 1.1 2011年6月14日 Group 改定 1.2 2011年7月13日 Group 改定 1.3 2011年7月27日 Group Atomic model / Database references ... Atomic model / Database references / Derived calculations / Non-polymer description / Structure summary 改定 1.4 2011年11月2日 Group 改定 1.5 2012年12月12日 Group 改定 1.6 2018年4月18日 Group / Other / カテゴリ / pdbx_database_statusItem / _pdbx_database_status.process_site改定 1.7 2023年8月9日 Group / Derived calculations / Refinement descriptionカテゴリ database_2 / pdbx_initial_refinement_model ... database_2 / pdbx_initial_refinement_model / struct_conn / struct_ref_seq_dif Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _struct_conn.pdbx_leaving_atom_flag / _struct_ref_seq_dif.details
すべて表示 表示を減らす
 データを開く
データを開く 基本情報
基本情報 要素
要素 キーワード
キーワード 機能・相同性情報
機能・相同性情報 HOMO SAPIENS (ヒト)
HOMO SAPIENS (ヒト) TOLYPOCLADIUM INFLATUM (菌類)
TOLYPOCLADIUM INFLATUM (菌類) X線回折 / PROTEIN STRUCTURE IS KNOWN IN THIS CELL / 解像度: 2 Å
X線回折 / PROTEIN STRUCTURE IS KNOWN IN THIS CELL / 解像度: 2 Å  データ登録者
データ登録者 引用
引用 ジャーナル: J.Mol.Biol. / 年: 1998
ジャーナル: J.Mol.Biol. / 年: 1998 構造の表示
構造の表示 Molmil
Molmil Jmol/JSmol
Jmol/JSmol ダウンロードとリンク
ダウンロードとリンク ダウンロード
ダウンロード 1cwm.cif.gz
1cwm.cif.gz PDBx/mmCIF形式
PDBx/mmCIF形式 pdb1cwm.ent.gz
pdb1cwm.ent.gz PDB形式
PDB形式 1cwm.json.gz
1cwm.json.gz PDBx/mmJSON形式
PDBx/mmJSON形式 その他のダウンロード
その他のダウンロード https://data.pdbj.org/pub/pdb/validation_reports/cw/1cwm
https://data.pdbj.org/pub/pdb/validation_reports/cw/1cwm ftp://data.pdbj.org/pub/pdb/validation_reports/cw/1cwm
ftp://data.pdbj.org/pub/pdb/validation_reports/cw/1cwm リンク
リンク 集合体
集合体
 要素
要素 HOMO SAPIENS (ヒト) / 遺伝子: CYCLOPHILIN / 遺伝子 (発現宿主): CYCLOPHILIN / 発現宿主:
HOMO SAPIENS (ヒト) / 遺伝子: CYCLOPHILIN / 遺伝子 (発現宿主): CYCLOPHILIN / 発現宿主: 
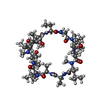
 タイプ: Cyclic peptide / クラス: 免疫抑制剤 / 分子量: 1220.625 Da / 分子数: 1 / Mutation: YES / 由来タイプ: 合成
タイプ: Cyclic peptide / クラス: 免疫抑制剤 / 分子量: 1220.625 Da / 分子数: 1 / Mutation: YES / 由来タイプ: 合成 TOLYPOCLADIUM INFLATUM (菌類) / 参照: NOR: NOR00033, CYCLOSPORIN A, 8 mutation
TOLYPOCLADIUM INFLATUM (菌類) / 参照: NOR: NOR00033, CYCLOSPORIN A, 8 mutation X線回折 / 使用した結晶の数: 1
X線回折 / 使用した結晶の数: 1  試料調製
試料調製 回転陽極 / タイプ: ENRAF-NONIUS FR571 / 波長: 1.5418
回転陽極 / タイプ: ENRAF-NONIUS FR571 / 波長: 1.5418  解析
解析 ムービー
ムービー コントローラー
コントローラー








































 PDBj
PDBj












