[English] 日本語
 Yorodumi
Yorodumi- PDB-1e1y: Flavopiridol inhibits glycogen phosphorylase by binding at the in... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1e1y | ||||||
|---|---|---|---|---|---|---|---|
| Title | Flavopiridol inhibits glycogen phosphorylase by binding at the inhibitor site | ||||||
 Components Components | GLYCOGEN PHOSPHORYLASE, MUSCLE FORM | ||||||
 Keywords Keywords | TRANSFERASE / ALLOSTERIC INHIBITION | ||||||
| Function / homology |  Function and homology information Function and homology informationglycogen phosphorylase / glycogen phosphorylase activity / glycogen catabolic process / skeletal muscle myofibril / pyridoxal phosphate binding / nucleotide binding Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.23 Å MOLECULAR REPLACEMENT / Resolution: 2.23 Å | ||||||
 Authors Authors | Oikonomakos, N.G. / Zographos, S.E. / Skamnaki, V.T. / Tsitsanou, K.E. / Johnson, L.N. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2000 Journal: J.Biol.Chem. / Year: 2000Title: Flavopiridol Inhibits Glycogen Phosphorylase by Binding at the Inhibitor Site Authors: Oikonomakos, N.G. / Schnier, J.B. / Zographos, S.E. / Skamnaki, V.T. / Tsitsanou, K.E. / Johnson, L.N. #1:  Journal: Protein Sci. / Year: 1999 Journal: Protein Sci. / Year: 1999Title: Allosteric Inhibition of Glycogen Phosphorylase a by the Potential Antidiabetic Drug 3-Isopropyl 4-(2-Chlorophenyl)-1,4-Dihydro-1-Ethyl-2-Methyl-Pyridine-3,5,6-Tricarboxylate Authors: Oikonomakos, N.G. / Tsitsanou, K.E. / Zographos, S.E. / Skamnaki, V.T. / Goldmann, S. / Bischoff, H. #2:  Journal: Structure / Year: 1997 Journal: Structure / Year: 1997Title: The Structure of Glycogen Phosphorylase B with an Alkyl-Dihydropyridine-Dicarboxylic Acid Compound, a Novel and Potent Inhibitor Authors: Zographos, S.E. / Oikonomakos, N.G. / Tsitsanou, K.E. / Leonidas, D.D. / Chrysina, E.D. / Skamnaki, V.T. / Bischoff, H. / Goldman, S. / Schramm, M. / Watson, K.A. / Johnson, L.N. | ||||||
| History |
| ||||||
| Remark 650 | HELIX DETERMINATION METHOD: AUTHOR PROVIDED. | ||||||
| Remark 700 | SHEET DETERMINATION METHOD: AUTHOR PROVIDED. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1e1y.cif.gz 1e1y.cif.gz | 192.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1e1y.ent.gz pdb1e1y.ent.gz | 151 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1e1y.json.gz 1e1y.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/e1/1e1y https://data.pdbj.org/pub/pdb/validation_reports/e1/1e1y ftp://data.pdbj.org/pub/pdb/validation_reports/e1/1e1y ftp://data.pdbj.org/pub/pdb/validation_reports/e1/1e1y | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1c8kC  1gfzC  2gpaS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein / Sugars , 2 types, 2 molecules A

| #1: Protein | Mass: 97291.203 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #4: Sugar | ChemComp-GLC / |
-Non-polymers , 4 types, 647 molecules 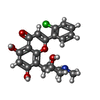




| #2: Chemical | ChemComp-CPB / |
|---|---|
| #3: Chemical | ChemComp-PO3 / |
| #5: Chemical | ChemComp-PLP / |
| #6: Water | ChemComp-HOH / |
-Details
| Sequence details | 2AMV SWALL P00489 1 - 13 NOT IN ATOMS LI REFERENCE: K.NAKANO, P.K.HWANG, R.J.FLETTERICK, FEBS LETT. ...2AMV SWALL P00489 1 - 13 NOT IN ATOMS LI REFERENCE: K.NAKANO, P.K.HWANG, R.J.FLETTERICK |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.4 Å3/Da / Density % sol: 48 % | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | pH: 6.7 Details: AS DESCRIBED PREVIOUSLY BY OIKONOMAKOS ET AL. (1999) PROTEIN SCIENCE 8, 1930-1945., pH 6.70 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Method: co-crystallization | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: X13 / Wavelength: 1.05 / Beamline: X13 / Wavelength: 1.05 |
| Detector | Date: Mar 15, 2000 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.05 Å / Relative weight: 1 |
| Reflection | Resolution: 2.23→29.9 Å / Num. obs: 40691 / % possible obs: 87.3 % / Observed criterion σ(I): 0 / Redundancy: 3.7 % / Rmerge(I) obs: 0.104 / Net I/σ(I): 11.1 |
| Reflection shell | Resolution: 2.23→2.27 Å / Rmerge(I) obs: 0.433 / Mean I/σ(I) obs: 2.4 / % possible all: 84.6 |
| Reflection | *PLUS Num. measured all: 321450 |
| Reflection shell | *PLUS % possible obs: 84.6 % |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2GPA Resolution: 2.23→29.9 Å / Cross valid method: FREE R-VALUE / σ(F): 0 Details: RESIDUES WHERE OVERALL -FACTOR VALUES EXCEED 60 A**2 INCLUDE 16-22 (71.1), 550-556 (69.4), AND 837-838 (75.9). TER PRO: RESIDUES 1-5,251-259,315-324,839- 842 WERE NOT DEFINED BY ELECTRON DENSITY
Refinement step | Cycle: LAST / Resolution: 2.23→29.9 Å |
Refine LS restraints |
Software | *PLUS Name:  X-PLOR / Version: 3.8 / Classification: refinement X-PLOR / Version: 3.8 / Classification: refinementRefine LS restraints | *PLUS
LS refinement shell | *PLUS Rfactor Rfree: 0.314 / Rfactor Rwork: 0.252 |
 Movie
Movie Controller
Controller


















































 PDBj
PDBj






