[English] 日本語
 Yorodumi
Yorodumi- PDB-4tno: Hypothetical protein PF1117 from Pyrococcus Furiosus: Structure s... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4tno | ||||||
|---|---|---|---|---|---|---|---|
| Title | Hypothetical protein PF1117 from Pyrococcus Furiosus: Structure solved by sulfur-SAD using Swiss Light Source Data | ||||||
 Components Components | CRISPR-associated endoribonuclease Cas2 | ||||||
 Keywords Keywords | HYDROLASE / SULFUR SAD | ||||||
| Function / homology |  Function and homology information Function and homology informationmaintenance of CRISPR repeat elements / RNA endonuclease activity / defense response to virus / Hydrolases; Acting on ester bonds / hydrolase activity / metal ion binding Similarity search - Function | ||||||
| Biological species |   Pyrococcus furiosus (archaea) Pyrococcus furiosus (archaea) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 2.14 Å SAD / Resolution: 2.14 Å | ||||||
 Authors Authors | Weinert, T. / Waltersperger, S. / Olieric, V. / Panepucci, E. / Chen, L. / Rose, J.P. / Wang, M. / Wang, B.C. / Southeast Collaboratory for Structural Genomics (SECSG) | ||||||
 Citation Citation |  Journal: Nat.Methods / Year: 2015 Journal: Nat.Methods / Year: 2015Title: Fast native-SAD phasing for routine macromolecular structure determination. Authors: Weinert, T. / Olieric, V. / Waltersperger, S. / Panepucci, E. / Chen, L. / Zhang, H. / Zhou, D. / Rose, J. / Ebihara, A. / Kuramitsu, S. / Li, D. / Howe, N. / Schnapp, G. / Pautsch, A. / ...Authors: Weinert, T. / Olieric, V. / Waltersperger, S. / Panepucci, E. / Chen, L. / Zhang, H. / Zhou, D. / Rose, J. / Ebihara, A. / Kuramitsu, S. / Li, D. / Howe, N. / Schnapp, G. / Pautsch, A. / Bargsten, K. / Prota, A.E. / Surana, P. / Kottur, J. / Nair, D.T. / Basilico, F. / Cecatiello, V. / Pasqualato, S. / Boland, A. / Weichenrieder, O. / Wang, B.C. / Steinmetz, M.O. / Caffrey, M. / Wang, M. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4tno.cif.gz 4tno.cif.gz | 31.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4tno.ent.gz pdb4tno.ent.gz | 19.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4tno.json.gz 4tno.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/tn/4tno https://data.pdbj.org/pub/pdb/validation_reports/tn/4tno ftp://data.pdbj.org/pub/pdb/validation_reports/tn/4tno ftp://data.pdbj.org/pub/pdb/validation_reports/tn/4tno | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4pgoC  4piiC  4r8tC  4r8uC  4tn8C  4wabC  4wauC  4wbqC  4wbxC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 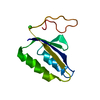
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 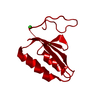
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 9992.677 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Pyrococcus furiosus (archaea) / Strain: ATCC 43587 / DSM 3638 / JCM 8422 / Vc1 / Gene: cas2, PF1117 / Production host: Pyrococcus furiosus (archaea) / Strain: ATCC 43587 / DSM 3638 / JCM 8422 / Vc1 / Gene: cas2, PF1117 / Production host:  References: UniProt: Q8U1T8, Hydrolases; Acting on ester bonds | ||
|---|---|---|---|
| #2: Chemical | | #3: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.45 Å3/Da / Density % sol: 49.77 % |
|---|---|
| Crystal grow | Temperature: 291 K / Method: vapor diffusion, hanging drop / pH: 7.3 Details: 1 MICROLITER DROPS CONTAINING EQUAL VOLUMES OF PROTEIN SOLUTION (10 MG/ML) AND A PRECIPITANT SOLUTION CONTAINING 0.1M SODIUM CITRATE BUFFER CONTAINING 0.1 M CHES |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06DA / Wavelength: 2.066 Å / Beamline: X06DA / Wavelength: 2.066 Å |
| Detector | Type: DECTRIS PILATUS 2M-F / Detector: PIXEL / Date: Aug 30, 2013 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 2.066 Å / Relative weight: 1 |
| Reflection | Resolution: 2.14→50 Å / Num. obs: 10426 / % possible obs: 97.1 % / Redundancy: 76.2 % / Rmerge(I) obs: 0.034 / Net I/σ(I): 71.7 |
| Reflection shell | Redundancy: 3 % / Rmerge(I) obs: 0.523 / Mean I/σ(I) obs: 2.2 / % possible all: 65.2 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  SAD / Resolution: 2.14→40.948 Å / SU ML: 0.42 / Cross valid method: FREE R-VALUE / σ(F): 1.46 / Phase error: 0.3861 / Stereochemistry target values: MLHL SAD / Resolution: 2.14→40.948 Å / SU ML: 0.42 / Cross valid method: FREE R-VALUE / σ(F): 1.46 / Phase error: 0.3861 / Stereochemistry target values: MLHL
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.14→40.948 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj


