[English] 日本語
 Yorodumi
Yorodumi- PDB-2ajy: Crystal Structure of Cocaine catalytic Antibody 7A1 Fab' in Compl... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2ajy | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal Structure of Cocaine catalytic Antibody 7A1 Fab' in Complex with ecgonine methyl ester and benzoic acid | ||||||
 Components Components | (Antibody 7A1 ...) x 2 | ||||||
 Keywords Keywords | IMMUNE SYSTEM / catalytic Antibody / Fab / ecgonine methyl ester / benzoic acid / HYDROLYTIC | ||||||
| Function / homology |  Function and homology information Function and homology informationimmune system process / immunoglobulin complex / B cell differentiation / extracellular region / metal ion binding / plasma membrane Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Zhu, X. / Wilson, I.A. | ||||||
 Citation Citation |  Journal: Structure / Year: 2006 Journal: Structure / Year: 2006Title: Complete reaction cycle of a cocaine catalytic antibody at atomic resolution. Authors: Zhu, X. / Dickerson, T.J. / Rogers, C.J. / Kaufmann, G.F. / Mee, J.M. / McKenzie, K.M. / Janda, K.D. / Wilson, I.A. | ||||||
| History |
| ||||||
| Remark 999 | SEQUENCE The sequence of these proteins are not available at any sequence database at the time of processing. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2ajy.cif.gz 2ajy.cif.gz | 103.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2ajy.ent.gz pdb2ajy.ent.gz | 78.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2ajy.json.gz 2ajy.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  2ajy_validation.pdf.gz 2ajy_validation.pdf.gz | 478.1 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  2ajy_full_validation.pdf.gz 2ajy_full_validation.pdf.gz | 485.7 KB | Display | |
| Data in XML |  2ajy_validation.xml.gz 2ajy_validation.xml.gz | 20.3 KB | Display | |
| Data in CIF |  2ajy_validation.cif.gz 2ajy_validation.cif.gz | 28.2 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/aj/2ajy https://data.pdbj.org/pub/pdb/validation_reports/aj/2ajy ftp://data.pdbj.org/pub/pdb/validation_reports/aj/2ajy ftp://data.pdbj.org/pub/pdb/validation_reports/aj/2ajy | HTTPS FTP |
-Related structure data
| Related structure data |  2ajsSC  2ajuC  2ajvC  2ajxC  2ajzC  2ak1C S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Antibody , 2 types, 2 molecules LH
| #1: Antibody | Mass: 24059.611 Da / Num. of mol.: 1 / Fragment: IMMUNOGLOBULIN IGG1 KAPPA LIGHT CHAIN / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #2: Antibody | Mass: 23865.783 Da / Num. of mol.: 1 / Fragment: IMMUNOGLOBULIN IGG1 HEAVY CHAIN / Source method: isolated from a natural source / Source: (natural)  |
-Non-polymers , 4 types, 182 molecules 
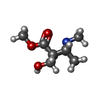
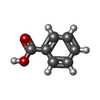




| #3: Chemical | ChemComp-ZN / #4: Chemical | ChemComp-ECG / | #5: Chemical | ChemComp-BEZ / | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3 Å3/Da / Density % sol: 59 % |
|---|---|
| Crystal grow | Temperature: 295 K / Method: vapor diffusion, sitting drop / pH: 5.5 Details: PEG 4000, zinc acetate, sodium acetate, pH 5.5, VAPOR DIFFUSION, SITTING DROP, temperature 295K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ALS ALS  / Beamline: 8.3.1 / Wavelength: 1.11587 Å / Beamline: 8.3.1 / Wavelength: 1.11587 Å |
| Detector | Type: ADSC QUANTUM 210 / Detector: CCD / Date: Nov 13, 2003 |
| Radiation | Monochromator: DOUBLE-CRYSTAL, Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.11587 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→50 Å / Num. obs: 34262 / % possible obs: 99.3 % / Observed criterion σ(I): -3 / Rmerge(I) obs: 0.074 / Χ2: 1.795 |
| Reflection shell | Resolution: 2.1→2.15 Å / % possible obs: 95.7 % / Rmerge(I) obs: 0.743 / Num. measured obs: 2180 / Χ2: 0.879 / % possible all: 95.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2AJS Resolution: 2.1→50 Å / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: Engh & Huber
| ||||||||||||||||||||||||||||||||
| Solvent computation | Bsol: 56.59 Å2 | ||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 46.389 Å2
| ||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.1→50 Å
| ||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller






















 PDBj
PDBj





