[English] 日本語
 Yorodumi
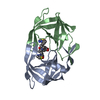
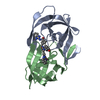
Yorodumi- PDB-4b3p: Structures of HIV-1 RT and RNA-DNA Complex Reveal a Unique RT Con... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4b3p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structures of HIV-1 RT and RNA-DNA Complex Reveal a Unique RT Conformation and Substrate Interface | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords | HYDROLASE/RNA/DNA / HYDROLASE-RNA-DNA COMPLEX / RNASE H / SUBUNIT INTERFACE / HYBRID | |||||||||
| Function / homology |  Function and homology information Function and homology informationintegrase activity / Integration of viral DNA into host genomic DNA / Autointegration results in viral DNA circles / Minus-strand DNA synthesis / Plus-strand DNA synthesis / Uncoating of the HIV Virion / 2-LTR circle formation / Vpr-mediated nuclear import of PICs / Early Phase of HIV Life Cycle / Integration of provirus ...integrase activity / Integration of viral DNA into host genomic DNA / Autointegration results in viral DNA circles / Minus-strand DNA synthesis / Plus-strand DNA synthesis / Uncoating of the HIV Virion / 2-LTR circle formation / Vpr-mediated nuclear import of PICs / Early Phase of HIV Life Cycle / Integration of provirus / APOBEC3G mediated resistance to HIV-1 infection / Binding and entry of HIV virion / viral life cycle / HIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / Assembly Of The HIV Virion / protein processing / Budding and maturation of HIV virion / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / RNA stem-loop binding / viral penetration into host nucleus / host multivesicular body / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / peptidase activity / host cell / viral nucleocapsid / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / DNA-directed DNA polymerase activity / symbiont-mediated suppression of host gene expression / viral translational frameshifting / symbiont entry into host cell / lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / DNA binding / zinc ion binding / identical protein binding Similarity search - Function | |||||||||
| Biological species |   HUMAN IMMUNODEFICIENCY VIRUS 1 HUMAN IMMUNODEFICIENCY VIRUS 1SYNTHETIC CONSTRUCT (others) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 4.839 Å MOLECULAR REPLACEMENT / Resolution: 4.839 Å | |||||||||
 Authors Authors | Lapkouski, M. / Tian, L. / Miller, J.T. / Le Grice, S.F.J. / Yang, W. | |||||||||
 Citation Citation |  Journal: Nat.Struct.Mol.Biol. / Year: 2013 Journal: Nat.Struct.Mol.Biol. / Year: 2013Title: Complexes of HIV-1 RT, Nnrti and RNA/DNA Hybrid Reveal a Structure Compatible with RNA Degradation Authors: Lapkouski, M. / Tian, L. / Miller, J.T. / Le Grice, S.F.J. / Yang, W. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4b3p.cif.gz 4b3p.cif.gz | 223.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4b3p.ent.gz pdb4b3p.ent.gz | 169.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4b3p.json.gz 4b3p.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/b3/4b3p https://data.pdbj.org/pub/pdb/validation_reports/b3/4b3p ftp://data.pdbj.org/pub/pdb/validation_reports/b3/4b3p ftp://data.pdbj.org/pub/pdb/validation_reports/b3/4b3p | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4b3oC  4b3qC  1fk9S  1rtdS  4b37 S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 64380.832 Da / Num. of mol.: 1 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)   HUMAN IMMUNODEFICIENCY VIRUS 1 / Production host: HUMAN IMMUNODEFICIENCY VIRUS 1 / Production host:  References: UniProt: P04585, RNA-directed DNA polymerase, DNA-directed DNA polymerase, retroviral ribonuclease H, HIV-1 retropepsin, exoribonuclease H |
|---|---|
| #2: Protein | Mass: 52973.785 Da / Num. of mol.: 1 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)   HUMAN IMMUNODEFICIENCY VIRUS 1 / Production host: HUMAN IMMUNODEFICIENCY VIRUS 1 / Production host:  References: UniProt: P04585, RNA-directed DNA polymerase, DNA-directed DNA polymerase, retroviral ribonuclease H, HIV-1 retropepsin |
| #3: DNA chain | Mass: 8962.752 Da / Num. of mol.: 1 / Fragment: PRIMER DNA / Source method: obtained synthetically / Source: (synth.) SYNTHETIC CONSTRUCT (others) |
| #4: RNA chain | Mass: 10646.402 Da / Num. of mol.: 1 / Fragment: TEMPLATE RNA / Source method: obtained synthetically / Source: (synth.) SYNTHETIC CONSTRUCT (others) |
| Sequence details | P66 MUTATIONS S68G, R83K, I411V, N447S, R461K, Y483H, D498A, V559I P51 MUTATIONS S68G, R83K, I411V, ...P66 MUTATIONS S68G, R83K, I411V, N447S, R461K, Y483H, D498A, V559I P51 MUTATIONS S68G, R83K, I411V, N-TERMINAL MRGSHHHHHH |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.93 Å3/Da / Density % sol: 62 % / Description: NONE |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion / pH: 6.5 Details: RT COMPLEX WAS MIXED WITH RESERVOIR SOLUTION CONTAINING 50MM SODIUM CACODYLATE PH6.5), 10MM MGCL2, 0.2M KCL, AND 10% PEG4000 (W/V). VAPOR DIFFUSION 4C. |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 22-BM / Wavelength: 1 / Beamline: 22-BM / Wavelength: 1 |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Apr 19, 2011 / Details: MIRRORS VERTICAL FOCUSING |
| Radiation | Monochromator: DOUBLE CRYSTAL - SI (220) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1 Å / Relative weight: 1 |
| Reflection | Resolution: 4.85→50 Å / Num. obs: 9097 / % possible obs: 99.7 % / Observed criterion σ(I): 2 / Redundancy: 11.6 % / Biso Wilson estimate: 185.31 Å2 / Rsym value: 0.16 / Net I/σ(I): 14.7 |
| Reflection shell | Resolution: 4.85→5.02 Å / Redundancy: 7.5 % / Mean I/σ(I) obs: 2.1 / Rsym value: 0.63 / % possible all: 99 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ID 1RTD, 1FK9 Resolution: 4.839→46.096 Å / SU ML: 1.06 / σ(F): 1.34 / Phase error: 50.33 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 185 Å2 | ||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 4.839→46.096 Å
| ||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller

























































































 PDBj
PDBj




































































