[English] 日本語
 Yorodumi
Yorodumi- PDB-1s1t: Crystal structure of L100I mutant HIV-1 reverse transcriptase in ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1s1t | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of L100I mutant HIV-1 reverse transcriptase in complex with UC-781 | ||||||
 Components Components | (Reverse transcriptase) x 2 | ||||||
 Keywords Keywords | TRANSFERASE / HIV-1 REVERSE TRANSCRIPTASE / AIDS / NNRTI / UC-781 / DRUG RESISTANCE MUTATIONS | ||||||
| Function / homology |  Function and homology information Function and homology informationintegrase activity / Integration of viral DNA into host genomic DNA / Autointegration results in viral DNA circles / Minus-strand DNA synthesis / Plus-strand DNA synthesis / Uncoating of the HIV Virion / 2-LTR circle formation / Vpr-mediated nuclear import of PICs / Early Phase of HIV Life Cycle / Integration of provirus ...integrase activity / Integration of viral DNA into host genomic DNA / Autointegration results in viral DNA circles / Minus-strand DNA synthesis / Plus-strand DNA synthesis / Uncoating of the HIV Virion / 2-LTR circle formation / Vpr-mediated nuclear import of PICs / Early Phase of HIV Life Cycle / Integration of provirus / APOBEC3G mediated resistance to HIV-1 infection / Binding and entry of HIV virion / viral life cycle / HIV-1 retropepsin / symbiont-mediated activation of host apoptosis / retroviral ribonuclease H / exoribonuclease H / exoribonuclease H activity / Assembly Of The HIV Virion / Budding and maturation of HIV virion / protein processing / viral genome integration into host DNA / establishment of integrated proviral latency / RNA-directed DNA polymerase / RNA stem-loop binding / viral penetration into host nucleus / host multivesicular body / RNA-directed DNA polymerase activity / RNA-DNA hybrid ribonuclease activity / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / peptidase activity / host cell / viral nucleocapsid / DNA recombination / DNA-directed DNA polymerase / aspartic-type endopeptidase activity / Hydrolases; Acting on ester bonds / DNA-directed DNA polymerase activity / symbiont-mediated suppression of host gene expression / viral translational frameshifting / symbiont entry into host cell / lipid binding / host cell nucleus / host cell plasma membrane / virion membrane / structural molecule activity / DNA binding / zinc ion binding / identical protein binding / membrane Similarity search - Function | ||||||
| Biological species |   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.4 Å MOLECULAR REPLACEMENT / Resolution: 2.4 Å | ||||||
 Authors Authors | Ren, J. / Nichols, C.E. / Chamberlain, P.P. / Stammers, D.K. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2004 Journal: J.Mol.Biol. / Year: 2004Title: Crystal structures of HIV-1 reverse transcriptases mutated at codons 100, 106 and 108 and mechanisms of resistance to non-nucleoside inhibitors Authors: Ren, J. / Nichols, C.E. / Chamberlain, P.P. / Weaver, K.L. / Short, S.A. / Stammers, D.K. #1:  Journal: J.VIROL. / Year: 2002 Journal: J.VIROL. / Year: 2002Title: Crystal structures of Zidovudine- or Lamivudine-resistant human immunodeficiency virus type 1 reverse transcriptases containing mutations at codons 41, 184, and 215 Authors: Chamberlain, P.P. / Ren, J. / Nichols, C.E. / Douglas, L. / Lennerstrand, J. / Larder, B.A. / Stuart, D.I. / Stammers, D.K. #2:  Journal: J.Mol.Biol. / Year: 2001 Journal: J.Mol.Biol. / Year: 2001Title: Structural Mechanisms of Drug Resistance for Mutations at Codons 181 and 188 in HIV-1 Reverse Transcriptase and the Improved Resilience of Second Generation Non-Nucleoside Inhibitors Authors: Ren, J. / Nichols, C. / Bird, L. / Chamberlain, P. / Weaver, K.L. / Short, S.A. / Stuart, D.I. / Stammers, D.K. #3:  Journal: J.Med.Chem. / Year: 2001 Journal: J.Med.Chem. / Year: 2001Title: 2-Amino-6-Arylsulfonylbenzonitriles as Non-Nucleoside reverse Transcriptase Inhibitors of HIV-1 Authors: Chan, J.H. / Hong, J.S. / Hunter III, R.N. / Orr, G.F. / Cowan, J.R. / Sherman, D.B. / Sparks, S.M. / Reitter, B.E. / Andrews III, C.W. / Hazen, R.J. / St Clair, M. / Boone, L.R. / Ferris, R. ...Authors: Chan, J.H. / Hong, J.S. / Hunter III, R.N. / Orr, G.F. / Cowan, J.R. / Sherman, D.B. / Sparks, S.M. / Reitter, B.E. / Andrews III, C.W. / Hazen, R.J. / St Clair, M. / Boone, L.R. / Ferris, R.G. / Creech, K.L. / Roberts, G.B. / Short, S.A. / Weaver, K. / Ott, R.J. / Ren, J. / Hopkins, A. / Stuart, D.I. / Stammers, D.K. #4:  Journal: Structure / Year: 2000 Journal: Structure / Year: 2000Title: Structural Basis for the Resilience of Efavirenz (Dmp-266) to Drug Resistance Mutations in HIV-1 Reverse Transcriptase Authors: Ren, J. / Milton, J. / Weaver, K.L. / Short, S.A. / Stuart, D.I. / Stammers, D.K. #5:  Journal: J.Biol.Chem. / Year: 2000 Journal: J.Biol.Chem. / Year: 2000Title: Binding of the Second Generation Non-Nucleoside Inhibitor S-1153 to HIV-1 Reverse Transcriptase Involves Extensive Main Chain Hydrogen Bonding Authors: Ren, J. / Nichols, C. / Bird, L.E. / Fujiwara, T. / Suginoto, H. / Stuart, D.I. / Stammers, D.K. #6:  Journal: J.Biol.Chem. / Year: 2000 Journal: J.Biol.Chem. / Year: 2000Title: Phenethylthiazolylthiourea (Pett) Non-Nucleoside Inhibitors of HIV-1 and HIV-2 Reverse Transcriptases. Structural and Biochemical Analyses Authors: Ren, J. / Diprose, J. / Warren, J. / Esnouf, R.M. / Bird, L.E. / Ikemizu, S. / Slater, M. / Milton, J. / Balzarini, J. / Stuart, D.I. / Stammers, D.K. #7:  Journal: J.Med.Chem. / Year: 1999 Journal: J.Med.Chem. / Year: 1999Title: Crystallographic Analysis of the Binding Modes of Thiazoloisoindolinone Non-Nucleoside Inhibitors to HIV-1 Reverse Transcriptase and Comparison with Modeling Studies Authors: Ren, J. / Esnouf, R.M. / Hopkins, A.L. / Stuart, D.I. / Stammers, D.K. #8:  Journal: J.Med.Chem. / Year: 1999 Journal: J.Med.Chem. / Year: 1999Title: Design of Mkc-442 (Emivirine) Analogues with Improved Activity Against Drug-Resistant HIV Mutants Authors: Hopkins, A.L. / Ren, J. / Tanaka, H. / Baba, M. / Okamato, M. / Stuart, D.I. / Stammers, D.K. #9:  Journal: Biochemistry / Year: 1998 Journal: Biochemistry / Year: 1998Title: Crystal Structures of HIV-1 Reverse Transcriptase in Complex with Carboxanilide Derivatives Authors: Ren, J. / Esnouf, R.M. / Hopkins, A.L. / Warren, J. / Balzarini, J. / Stuart, D.I. / Stammers, D.K. #10:  Journal: Proc.Natl.Acad.Sci.USA / Year: 1998 Journal: Proc.Natl.Acad.Sci.USA / Year: 1998Title: 3'-Azido-3'-Deoxythymidine Drug Resistance Mutations in HIV-1 Reverse Transcriptase Can Induce Long Range Conformational Changes Authors: Ren, J. / Esnouf, R.M. / Hopkins, A.L. / Jones, E.Y. / Kirby, I. / Keeling, J. / Ross, C.K. / Larder, B.A. / Stuart, D.I. / Stammers, D.K. #11:  Journal: Acta Crystallogr.,Sect.D / Year: 1998 Journal: Acta Crystallogr.,Sect.D / Year: 1998Title: Continuous and Discontinuous Changes in the Unit Cell of HIV-1 Reverse Transcriptase Crystals on Dehydration Authors: Esnouf, R.M. / Ren, J. / Garman, E. / Somers, D.O. / Ross, C.K. / Jones, E.Y. / Stammers, D.K. / Stuart, D.I. #12:  Journal: Proc.Natl.Acad.Sci.USA / Year: 1997 Journal: Proc.Natl.Acad.Sci.USA / Year: 1997Title: Unique Features in the Structure of the Complex between HIV-1 Reverse Transcriptase and the Bis(Heteroaryl)Piperazine (Bhap) U-90152 Explain Resistance Mutations for This Non-Nucleoside Inhibitor Authors: Esnouf, R.M. / Ren, J. / Hopkins, A.L. / Ross, C.K. / Jones, E.Y. / Stammers, D.K. / Stuart, D.I. #13:  Journal: J.Med.Chem. / Year: 1996 Journal: J.Med.Chem. / Year: 1996Title: Complexes of HIV-1 Reverse Transcriptase with Inhibitors of the HEPT Series Reveal Conformational Changes Relevant to the Design of Potent Non-Nucleoside Inhibitors Authors: L Hopkins, A. / Ren, J. / Esnouf, R.M. / Willcox, B.E. / Jones, E.Y. / Ross, C.K. / Miyasaka, T. / Walker, R.T. / Tanaka, H. / Stammers, D.K. / Stuart, D.I. #14:  Journal: Structure / Year: 1995 Journal: Structure / Year: 1995Title: The Structure of HIV-1 Reverse Transcriptase Complexed with 9-Chloro-TIBO: Lessons for Inhibitor Design Authors: Ren, J. / Esnouf, R.M. / Hopkins, A.L. / Ross, C.K. / Jones, E.Y. / Stammers, D.K. / Stuart, D.I. #15:  Journal: Nat.Struct.Biol. / Year: 1995 Journal: Nat.Struct.Biol. / Year: 1995Title: High Resolution Structures of HIV-1 RT from Four RT-Inhibitor Complexes Authors: Ren, J. / Esnouf, R.M. / Garman, E. / Somers, D.O. / Ross, C.K. / Kirby, I. / Keeling, J. / Darby, G. / Jones, E.Y. / Stuart, D.I. / Stammers, D.K. #16:  Journal: Nat.Struct.Biol. / Year: 1995 Journal: Nat.Struct.Biol. / Year: 1995Title: Mechanism of Inhibition of HIV-1 Reverse Transcriptase by Non-Nucleoside Inhibitors Authors: Esnouf, R.M. / Ren, J. / Ross, C.K. / Jones, E.Y. / Stammers, D.K. / Stuart, D.I. #17:  Journal: J.Mol.Biol. / Year: 1994 Journal: J.Mol.Biol. / Year: 1994Title: Crystals of HIV-1 Reverse Transcriptase Diffracting to 2.2 A Resolution Authors: Stammers, D.K. / Somers, D.O. / Ross, C.K. / Kirby, I. / Ray, P.H. / Wilson, J.E. / Norman, M. / Ren, J. / Esnouf, R.M. / Garman, E. / Jones, E.Y. / Stuart, D.I. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1s1t.cif.gz 1s1t.cif.gz | 212.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1s1t.ent.gz pdb1s1t.ent.gz | 168.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1s1t.json.gz 1s1t.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/s1/1s1t https://data.pdbj.org/pub/pdb/validation_reports/s1/1s1t ftp://data.pdbj.org/pub/pdb/validation_reports/s1/1s1t ftp://data.pdbj.org/pub/pdb/validation_reports/s1/1s1t | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 64594.949 Da / Num. of mol.: 1 / Fragment: P66 / Mutation: L100I Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Human immunodeficiency virus 1 / Genus: Lentivirus / Gene: POL / Plasmid: pkk233-2 / Production host: Human immunodeficiency virus 1 / Genus: Lentivirus / Gene: POL / Plasmid: pkk233-2 / Production host:  | ||||||
|---|---|---|---|---|---|---|---|
| #2: Protein | Mass: 51399.047 Da / Num. of mol.: 1 / Fragment: P51 / Mutation: L100I Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Human immunodeficiency virus 1 / Genus: Lentivirus / Gene: POL / Plasmid: pkk233-2 / Production host: Human immunodeficiency virus 1 / Genus: Lentivirus / Gene: POL / Plasmid: pkk233-2 / Production host:  | ||||||
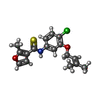
| #3: Chemical | | #4: Chemical | ChemComp-UC1 / | #5: Water | ChemComp-HOH / | Has protein modification | Y | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.42 Å3/Da / Density % sol: 48.75 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 5 Details: pH 5, VAPOR DIFFUSION, SITTING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM14 / Wavelength: 0.918 Å / Beamline: BM14 / Wavelength: 0.918 Å |
| Detector | Type: MARRESEARCH / Detector: IMAGE PLATE / Date: Dec 16, 1997 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.918 Å / Relative weight: 1 |
| Reflection | Resolution: 2.4→30 Å / Num. obs: 41571 / % possible obs: 96.3 % / Observed criterion σ(I): -1.5 / Redundancy: 4.84 % / Biso Wilson estimate: 53.4 Å2 / Rmerge(I) obs: 0.067 / Net I/σ(I): 13.8 |
| Reflection shell | Resolution: 2.4→2.49 Å / Redundancy: 2.85 % / Rmerge(I) obs: 0.31 / Mean I/σ(I) obs: 1.9 / Num. unique all: 3398 / % possible all: 80.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT / Resolution: 2.4→29.42 Å / Rfactor Rfree error: 0.006 / Data cutoff high absF: 2097493.93 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 MOLECULAR REPLACEMENT / Resolution: 2.4→29.42 Å / Rfactor Rfree error: 0.006 / Data cutoff high absF: 2097493.93 / Data cutoff low absF: 0 / Isotropic thermal model: RESTRAINED / Cross valid method: THROUGHOUT / σ(F): 0 Details: THE STANDARD CRYSTALLOGRAPHIC RESIDUAL WAS USED AS TARGET FUNCTION IN THE FINAL ROUND OF REFINEMENT INCLUDING ALL THE REFLECTIONS INSTEAD OF THE MLF IN THE PREVIOUS ROUNDS OF REFINEMENT WITH ...Details: THE STANDARD CRYSTALLOGRAPHIC RESIDUAL WAS USED AS TARGET FUNCTION IN THE FINAL ROUND OF REFINEMENT INCLUDING ALL THE REFLECTIONS INSTEAD OF THE MLF IN THE PREVIOUS ROUNDS OF REFINEMENT WITH CROSS-VALIDATION, WHICH RESULTED IN THE R-FACTOR FOR ALL DATA BEING SMALLER THAN R-WORKING.
| ||||||||||||||||||||||||||||||||||||
| Solvent computation | Solvent model: FLAT MODEL / Bsol: 46.3121 Å2 / ksol: 0.336477 e/Å3 | ||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 53.7 Å2
| ||||||||||||||||||||||||||||||||||||
| Refine analyze |
| ||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.4→29.42 Å
| ||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.4→2.49 Å / Rfactor Rfree error: 0.029 / Total num. of bins used: 10
| ||||||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller































































 PDBj
PDBj