+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31192 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human Mediator (deletion of MED1-IDR) Head module | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.8 Å | |||||||||
 Authors Authors | Yin X / Li J / Wu Z / Liu W / Xu Y | |||||||||
 Citation Citation |  Journal: Science / Year: 2021 Journal: Science / Year: 2021Title: Structures of the human Mediator and Mediator-bound preinitiation complex. Authors: Xizi Chen / Xiaotong Yin / Jiabei Li / Zihan Wu / Yilun Qi / Xinxin Wang / Weida Liu / Yanhui Xu /  Abstract: The 1.3-megadalton transcription factor IID (TFIID) is required for preinitiation complex (PIC) assembly and RNA polymerase II (Pol II)-mediated transcription initiation on almost all genes. The 26- ...The 1.3-megadalton transcription factor IID (TFIID) is required for preinitiation complex (PIC) assembly and RNA polymerase II (Pol II)-mediated transcription initiation on almost all genes. The 26-subunit Mediator stimulates transcription and cyclin-dependent kinase 7 (CDK7)-mediated phosphorylation of the Pol II C-terminal domain (CTD). We determined the structures of human Mediator in the Tail module-extended (at near-atomic resolution) and Tail-bent conformations and structures of TFIID-based PIC-Mediator (76 polypeptides, ~4.1 megadaltons) in four distinct conformations. PIC-Mediator assembly induces concerted reorganization (Head-tilting and Middle-down) of Mediator and creates a Head-Middle sandwich, which stabilizes two CTD segments and brings CTD to CDK7 for phosphorylation; this suggests a CTD-gating mechanism favorable for phosphorylation. The TFIID-based PIC architecture modulates Mediator organization and TFIIH stabilization, underscoring the importance of TFIID in orchestrating PIC-Mediator assembly. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31192.map.gz emd_31192.map.gz | 29.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31192-v30.xml emd-31192-v30.xml emd-31192.xml emd-31192.xml | 12.6 KB 12.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_31192.png emd_31192.png | 113.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31192 http://ftp.pdbj.org/pub/emdb/structures/EMD-31192 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31192 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31192 | HTTPS FTP |
-Validation report
| Summary document |  emd_31192_validation.pdf.gz emd_31192_validation.pdf.gz | 295.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_31192_full_validation.pdf.gz emd_31192_full_validation.pdf.gz | 295.3 KB | Display | |
| Data in XML |  emd_31192_validation.xml.gz emd_31192_validation.xml.gz | 7.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31192 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31192 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31192 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31192 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_31192.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31192.map.gz / Format: CCP4 / Size: 512 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.054 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
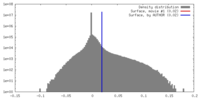
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Structure of the human Mediator complex
| Entire | Name: Structure of the human Mediator complex |
|---|---|
| Components |
|
-Supramolecule #1: Structure of the human Mediator complex
| Supramolecule | Name: Structure of the human Mediator complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#27 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant cell: Expi293 Homo sapiens (human) / Recombinant cell: Expi293 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Support film - Material: CARBON / Support film - topology: HOLEY |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.8 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 400082 |
|---|---|
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|
 Movie
Movie Controller
Controller






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)