+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-6771 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of human respiratory complex I matrix arm | |||||||||
 Map data Map data | This map was obtained by sub-region refinemet | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationComplex I biogenesis / protein lipoylation / Mitochondrial Fatty Acid Beta-Oxidation / Protein lipoylation / mitochondrial [2Fe-2S] assembly complex / Respiratory electron transport / protein insertion into mitochondrial inner membrane / blastocyst hatching / ubiquinone-6 biosynthetic process / cellular response to oxygen levels ...Complex I biogenesis / protein lipoylation / Mitochondrial Fatty Acid Beta-Oxidation / Protein lipoylation / mitochondrial [2Fe-2S] assembly complex / Respiratory electron transport / protein insertion into mitochondrial inner membrane / blastocyst hatching / ubiquinone-6 biosynthetic process / cellular response to oxygen levels / iron-sulfur cluster assembly complex / : / mitochondrial large ribosomal subunit binding / gliogenesis / oxidoreductase activity, acting on NAD(P)H, quinone or similar compound as acceptor / neural precursor cell proliferation / cardiac muscle tissue development / [2Fe-2S] cluster assembly / oxygen sensor activity / cellular respiration / sodium ion transport / iron-sulfur cluster assembly / NADH:ubiquinone reductase (H+-translocating) / positive regulation of execution phase of apoptosis / proton motive force-driven mitochondrial ATP synthesis / NADH dehydrogenase activity / mitochondrial ATP synthesis coupled electron transport / respiratory chain complex I / : / mitochondrial electron transport, NADH to ubiquinone / mitochondrial respiratory chain complex I assembly / endopeptidase activator activity / RHOG GTPase cycle / electron transport coupled proton transport / acyl binding / acyl carrier activity / NADH dehydrogenase (ubiquinone) activity / cellular response to interferon-beta / quinone binding / cellular response to retinoic acid / extrinsic apoptotic signaling pathway / response to cAMP / Mitochondrial protein degradation / substantia nigra development / aerobic respiration / neurogenesis / respiratory electron transport chain / reactive oxygen species metabolic process / synaptic membrane / fatty acid binding / mitochondrial membrane / brain development / regulation of protein phosphorylation / mitochondrial intermembrane space / fatty acid biosynthetic process / 2 iron, 2 sulfur cluster binding / circadian rhythm / positive regulation of protein catabolic process / NAD binding / FMN binding / nervous system development / 4 iron, 4 sulfur cluster binding / protease binding / response to oxidative stress / mitochondrial inner membrane / electron transfer activity / nuclear body / mitochondrial matrix / negative regulation of DNA-templated transcription / neuronal cell body / ubiquitin protein ligase binding / calcium ion binding / protein-containing complex binding / mitochondrion / RNA binding / nucleoplasm / ATP binding / nucleus / metal ion binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.4 Å | |||||||||
 Authors Authors | Gu J / Wu M / Yang M | |||||||||
 Citation Citation |  Journal: Cell / Year: 2017 Journal: Cell / Year: 2017Title: Architecture of Human Mitochondrial Respiratory Megacomplex IIIIIV. Authors: Runyu Guo / Shuai Zong / Meng Wu / Jinke Gu / Maojun Yang /  Abstract: The respiratory megacomplex represents the highest-order assembly of respiratory chain complexes, and it allows mitochondria to respond to energy-requiring conditions. To understand its architecture, ...The respiratory megacomplex represents the highest-order assembly of respiratory chain complexes, and it allows mitochondria to respond to energy-requiring conditions. To understand its architecture, we examined the human respiratory chain megacomplex-IIIIIV (MCIIIIIV) with 140 subunits and a subset of associated cofactors using cryo-electron microscopy. The MCIIIIIV forms a circular structure with the dimeric CIII located in the center, where it is surrounded by two copies each of CI and CIV. Two cytochrome c (Cyt.c) molecules are positioned to accept electrons on the surface of the c state CIII dimer. Analyses indicate that CII could insert into the gaps between CI and CIV to form a closed ring, which we termed the electron transport chain supercomplex. The structure not only reveals the precise assignment of individual subunits of human CI and CIII, but also enables future in-depth analysis of the electron transport chain as a whole. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_6771.map.gz emd_6771.map.gz | 21.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-6771-v30.xml emd-6771-v30.xml emd-6771.xml emd-6771.xml | 10.9 KB 10.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_6771.png emd_6771.png | 15.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-6771 http://ftp.pdbj.org/pub/emdb/structures/EMD-6771 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6771 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-6771 | HTTPS FTP |
-Validation report
| Summary document |  emd_6771_validation.pdf.gz emd_6771_validation.pdf.gz | 305 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_6771_full_validation.pdf.gz emd_6771_full_validation.pdf.gz | 304.6 KB | Display | |
| Data in XML |  emd_6771_validation.xml.gz emd_6771_validation.xml.gz | 7.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6771 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6771 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6771 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-6771 | HTTPS FTP |
-Related structure data
| Related structure data |  5xtbMC  6772C  6773C  6774C  6775C  6776C  5xtcC  5xtdC  5xteC  5xthC  5xtiC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_6771.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_6771.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | This map was obtained by sub-region refinemet | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.083 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
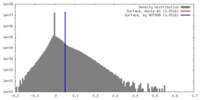
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Human respiratory complex I matrix arm
| Entire | Name: Human respiratory complex I matrix arm |
|---|---|
| Components |
|
-Supramolecule #1: Human respiratory complex I matrix arm
| Supramolecule | Name: Human respiratory complex I matrix arm / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#18 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.4 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 1.25 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| CTF correction | Software - Name: CTFFIND (ver. 3.0) |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.4 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: RELION (ver. 1.4) / Number images used: 167761 |
| Initial angle assignment | Type: RANDOM ASSIGNMENT / Software - Name: RELION (ver. 1.4) |
| Final angle assignment | Type: RANDOM ASSIGNMENT / Software - Name: RELION (ver. 1.4) |
 Movie
Movie Controller
Controller
























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)