[English] 日本語
 Yorodumi
Yorodumi- PDB-2vuk: Structure of the p53 core domain mutant Y220C bound to the stabil... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2vuk | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of the p53 core domain mutant Y220C bound to the stabilizing small-molecule drug PhiKan083 | ||||||
 Components Components | CELLULAR TUMOR ANTIGEN P53 | ||||||
 Keywords Keywords | TRANSCRIPTION / METAL BINDING / PHOSPHOPROTEIN / UBL CONJUGATION / ACTIVATOR / CELL CYCLE / ACETYLATION / METHYLATION / ZINC / CANCER / NUCLEUS / APOPTOSIS / CYTOPLASM / TUMOR SUPPRESSOR / VIRTUAL SCREENING / SECOND-SITE SUPPRESSOR MUTATION / COVALENT PROTEIN-RNA LINKAGE / SMALL-MOLECULE DRUG / ALTERNATIVE SPLICING / P53 DNA- BINDING DOMAIN / TRANSCRIPTION REGULATION / NUCLEAR PROTEIN / SURFACE CREVICE / DISEASE MUTATION / PROTEIN STABILIZATION / HOST-VIRUS INTERACTION / LI-FRAUMENI SYNDROME / ENDOPLASMIC RETICULUM / METAL-BINDING / ANTI-ONCOGENE / SUPERSTABLE MUTANT / DNA-BINDING PROTEIN / DNA BINDING / DNA-BINDING / POLYMORPHISM / GLYCOPROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of helicase activity / Loss of function of TP53 in cancer due to loss of tetramerization ability / Regulation of TP53 Expression / signal transduction by p53 class mediator / negative regulation of G1 to G0 transition / negative regulation of glucose catabolic process to lactate via pyruvate / Transcriptional activation of cell cycle inhibitor p21 / regulation of intrinsic apoptotic signaling pathway by p53 class mediator / negative regulation of pentose-phosphate shunt / ATP-dependent DNA/DNA annealing activity ...negative regulation of helicase activity / Loss of function of TP53 in cancer due to loss of tetramerization ability / Regulation of TP53 Expression / signal transduction by p53 class mediator / negative regulation of G1 to G0 transition / negative regulation of glucose catabolic process to lactate via pyruvate / Transcriptional activation of cell cycle inhibitor p21 / regulation of intrinsic apoptotic signaling pathway by p53 class mediator / negative regulation of pentose-phosphate shunt / ATP-dependent DNA/DNA annealing activity / Activation of NOXA and translocation to mitochondria / regulation of cell cycle G2/M phase transition / oligodendrocyte apoptotic process / negative regulation of miRNA processing / intrinsic apoptotic signaling pathway in response to hypoxia / positive regulation of thymocyte apoptotic process / oxidative stress-induced premature senescence / regulation of tissue remodeling / positive regulation of mitochondrial membrane permeability / regulation of fibroblast apoptotic process / mRNA transcription / bone marrow development / circadian behavior / positive regulation of programmed necrotic cell death / T cell proliferation involved in immune response / regulation of mitochondrial membrane permeability involved in apoptotic process / histone deacetylase regulator activity / RUNX3 regulates CDKN1A transcription / germ cell nucleus / homolactic fermentation / TP53 Regulates Transcription of Death Receptors and Ligands / TP53 regulates transcription of additional cell cycle genes whose exact role in the p53 pathway remain uncertain / Activation of PUMA and translocation to mitochondria / Regulation of TP53 Activity through Association with Co-factors / regulation of DNA damage response, signal transduction by p53 class mediator / negative regulation of glial cell proliferation / negative regulation of neuroblast proliferation / mitochondrial DNA repair / T cell lineage commitment / Formation of Senescence-Associated Heterochromatin Foci (SAHF) / thymocyte apoptotic process / ER overload response / TP53 Regulates Transcription of Caspase Activators and Caspases / cardiac septum morphogenesis / entrainment of circadian clock by photoperiod / B cell lineage commitment / negative regulation of mitophagy / negative regulation of DNA replication / Zygotic genome activation (ZGA) / Association of TriC/CCT with target proteins during biosynthesis / PI5P Regulates TP53 Acetylation / TP53 Regulates Transcription of Genes Involved in Cytochrome C Release / necroptotic process / positive regulation of release of cytochrome c from mitochondria / negative regulation of telomere maintenance via telomerase / SUMOylation of transcription factors / TP53 regulates transcription of several additional cell death genes whose specific roles in p53-dependent apoptosis remain uncertain / TFIID-class transcription factor complex binding / cellular response to actinomycin D / intrinsic apoptotic signaling pathway by p53 class mediator / negative regulation of reactive oxygen species metabolic process / rRNA transcription / Transcriptional Regulation by VENTX / cellular response to UV-C / viral process / general transcription initiation factor binding / replicative senescence / intrinsic apoptotic signaling pathway in response to endoplasmic reticulum stress / positive regulation of RNA polymerase II transcription preinitiation complex assembly / neuroblast proliferation / intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / Pyroptosis / positive regulation of execution phase of apoptosis / embryonic organ development / hematopoietic stem cell differentiation / chromosome organization / type II interferon-mediated signaling pathway / response to X-ray / TP53 Regulates Transcription of Genes Involved in G1 Cell Cycle Arrest / somitogenesis / hematopoietic progenitor cell differentiation / positive regulation of cardiac muscle cell apoptotic process / core promoter sequence-specific DNA binding / glial cell proliferation / negative regulation of stem cell proliferation / cellular response to glucose starvation / mitophagy / negative regulation of fibroblast proliferation / cis-regulatory region sequence-specific DNA binding / Regulation of TP53 Activity through Acetylation / positive regulation of intrinsic apoptotic signaling pathway / 14-3-3 protein binding / negative regulation of proteolysis / response to salt stress / mitotic G1 DNA damage checkpoint signaling / cardiac muscle cell apoptotic process / transcription repressor complex / gastrulation / MDM2/MDM4 family protein binding / transforming growth factor beta receptor signaling pathway Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.5 Å MOLECULAR REPLACEMENT / Resolution: 1.5 Å | ||||||
 Authors Authors | Joerger, A.C. / Boeckler, F.M. / Fersht, A.R. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.USA / Year: 2008 Journal: Proc.Natl.Acad.Sci.USA / Year: 2008Title: Targeted Rescue of a Destabilized Mutant of P53 by an in Silico Screened Drug. Authors: Boeckler, F.M. / Joerger, A.C. / Jaggi, G. / Rutherford, T.J. / Veprintsev, D.B. / Fersht, A.R. #1:  Journal: Proc.Natl.Acad.Sci.USA / Year: 2006 Journal: Proc.Natl.Acad.Sci.USA / Year: 2006Title: Structural Basis for Understanding Oncogenic P53 Mutations and Designing Rescue Drugs Authors: Joerger, A.C. / Ang, H.-C. / Fersht, A.R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2vuk.cif.gz 2vuk.cif.gz | 104 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2vuk.ent.gz pdb2vuk.ent.gz | 78.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2vuk.json.gz 2vuk.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/vu/2vuk https://data.pdbj.org/pub/pdb/validation_reports/vu/2vuk ftp://data.pdbj.org/pub/pdb/validation_reports/vu/2vuk ftp://data.pdbj.org/pub/pdb/validation_reports/vu/2vuk | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2j1xS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
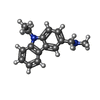
| #1: Protein | Mass: 24530.811 Da / Num. of mol.: 2 / Fragment: DNA-BINDING DOMAIN, RESIDUES 94-312 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Production host: HOMO SAPIENS (human) / Production host:  #2: Chemical | #3: Chemical | ChemComp-P83 / | #4: Water | ChemComp-HOH / | Compound details | ENGINEERED RESIDUE IN CHAIN A, MET 133 TO LEU ENGINEERED RESIDUE IN CHAIN A, VAL 203 TO ALA ...ENGINEERED | Nonpolymer details | 1-(9-ETHYL-9H-CARBAZOL-3-YL)-N-METHYLMETHANAMINE (P83): STABILIZING SMALL-MOLECULE COMPOUND, ...1-(9-ETHYL-9H-CARBAZOL-3-YL)-N-METHYLMETH | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.5 Å3/Da / Density % sol: 51 % / Description: NONE |
|---|---|
| Crystal grow | Temperature: 294 K / Method: vapor diffusion, sitting drop / pH: 7.2 Details: SITTING DROP VAPOR DIFFUSION AT 21 DEGREE C. PROTEIN SOLUTION: 6 MG/ML PROTEIN IN 25 MM SODIUM PHOSPHATE PH 7.2, 150 MM KCL, 5 MM DTT. RESERVOIR BUFFER: 100 MM HEPES PH 7.2, 19 % PEG 4000, 5 ...Details: SITTING DROP VAPOR DIFFUSION AT 21 DEGREE C. PROTEIN SOLUTION: 6 MG/ML PROTEIN IN 25 MM SODIUM PHOSPHATE PH 7.2, 150 MM KCL, 5 MM DTT. RESERVOIR BUFFER: 100 MM HEPES PH 7.2, 19 % PEG 4000, 5 MM DTT. CRYSTALS WERE SOAKED WITH 10 MM PHIKAN083 IN CRYO BUFFER CONTAINING 100 MM HEPES PH 7.2, 10 MM SODIUM PHOSPHATE PH 7.2, 19 % PEG 4000, 20 % GLYCEROL, 150 MM KCL. |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 0.9699 / Beamline: I04 / Wavelength: 0.9699 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: Dec 7, 2007 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9699 Å / Relative weight: 1 |
| Reflection | Resolution: 1.5→65.1 Å / Num. obs: 76025 / % possible obs: 96.6 % / Redundancy: 5.6 % / Biso Wilson estimate: 13.8 Å2 / Rmerge(I) obs: 0.07 / Net I/σ(I): 17.7 |
| Reflection shell | Resolution: 1.5→1.58 Å / Redundancy: 4.6 % / Rmerge(I) obs: 0.23 / Mean I/σ(I) obs: 5.6 / % possible all: 83.4 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2J1X Resolution: 1.5→65.1 Å / Cross valid method: THROUGHOUT
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 15.9 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.5→65.1 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller

























































 PDBj
PDBj