[English] 日本語
 Yorodumi
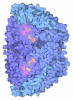
Yorodumi- PDB-7b1s: Crystal structure of the ethyl-coenzyme M reductase from Candidat... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7b1s | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the ethyl-coenzyme M reductase from Candidatus Ethanoperedens thermophilum at 0.994-A resolution | |||||||||
 Components Components | (Ethyl-Coenzyme M reductase ...) x 3 | |||||||||
 Keywords Keywords | TRANSFERASE / Ethyl-CoM reductase / Methyl-CoM reductase / ethane-oxidizers / F430-cofactor / post-translational modification / gas channel / coenzyme M / coenzyme B / true atomic resolution / thermophile / archaea. | |||||||||
| Function / homology | 1-THIOETHANESULFONIC ACID / : / : / Coenzyme B / Dimethylated-F430 cofactor / Chem-UUT Function and homology information Function and homology information | |||||||||
| Biological species |  Candidatus Ethanoperedens thermophilum (archaea) Candidatus Ethanoperedens thermophilum (archaea) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 0.992 Å MOLECULAR REPLACEMENT / Resolution: 0.992 Å | |||||||||
 Authors Authors | Wagner, T. / Lemaire, O.N. / Engilberge, S. | |||||||||
| Funding support |  Germany, 2items Germany, 2items
| |||||||||
 Citation Citation |  Journal: Science / Year: 2021 Journal: Science / Year: 2021Title: Crystal structure of a key enzyme for anaerobic ethane activation. Authors: Hahn, C.J. / Lemaire, O.N. / Kahnt, J. / Engilberge, S. / Wegener, G. / Wagner, T. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7b1s.cif.gz 7b1s.cif.gz | 1.2 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7b1s.ent.gz pdb7b1s.ent.gz | 975.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7b1s.json.gz 7b1s.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/b1/7b1s https://data.pdbj.org/pub/pdb/validation_reports/b1/7b1s ftp://data.pdbj.org/pub/pdb/validation_reports/b1/7b1s ftp://data.pdbj.org/pub/pdb/validation_reports/b1/7b1s | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7b2cC  7b2hC  5a8wS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Ethyl-Coenzyme M reductase ... , 3 types, 6 molecules ADBECF
| #1: Protein | Mass: 66344.344 Da / Num. of mol.: 2 / Source method: isolated from a natural source Details: Seven post-translational modifications exist in this subunit: N1-methylhistidine291 5(S)-methylarginine305 S-methylcysteine354 3-methylisoleucine377 2(S)-methylglutamine445 Thioglycine490 N2-methylhistidine491 Source: (natural)  Candidatus Ethanoperedens thermophilum (archaea) Candidatus Ethanoperedens thermophilum (archaea)Cell line: / / Organ: / / Plasmid details: Enrichment culture / Variant: / / Tissue: / / References: coenzyme-B sulfoethylthiotransferase #2: Protein | Mass: 49874.453 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  Candidatus Ethanoperedens thermophilum (archaea) Candidatus Ethanoperedens thermophilum (archaea)Cell line: / / Organ: / / Plasmid details: Enrichment culture / Variant: / / Tissue: / / References: coenzyme-B sulfoethylthiotransferase #3: Protein | Mass: 30578.789 Da / Num. of mol.: 2 / Source method: isolated from a natural source Source: (natural)  Candidatus Ethanoperedens thermophilum (archaea) Candidatus Ethanoperedens thermophilum (archaea)Cell line: / / Organ: / / Plasmid details: Enrichment culture / Variant: / / Tissue: / / References: coenzyme-B sulfoethylthiotransferase |
|---|
-Non-polymers , 10 types, 3111 molecules 
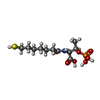





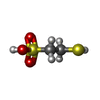











| #4: Chemical | ChemComp-GOL / #5: Chemical | #6: Chemical | ChemComp-MN / | #7: Chemical | ChemComp-K / #8: Chemical | ChemComp-CL / #9: Chemical | #10: Chemical | #11: Chemical | #12: Chemical | #13: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.27 Å3/Da / Density % sol: 45.87 % / Description: Yellow brick of 250 um long. |
|---|---|
| Crystal grow | Temperature: 293.15 K / Method: vapor diffusion, sitting drop / pH: 8.5 Details: Crystals were obtained by initial screening at 20 degree celsius using the sitting drop method on a 96-Well MRC 2-Drop Crystallization Plates in polystyrene (SWISSCI). The crystallization ...Details: Crystals were obtained by initial screening at 20 degree celsius using the sitting drop method on a 96-Well MRC 2-Drop Crystallization Plates in polystyrene (SWISSCI). The crystallization reservoir contained 90 ul of mother liquor, crystallization drop contained a mixture of 0.6 ul protein at 16.22 mg.ml-1 and 0.6 ul precipitant. The crystal was obtained by initial screening using the JBScreen Pentaerythritol screen from Jena Bioscience in a Coy tent under an N2/H2 atmosphere (95:5 %). The crystallization reservoir contained 45 % (w/v) Pentaerythritol Propoxylate (5/4 PO/OH), 100 mM Tris pH 8.5 and 400 mM potassium chloride. PH range: 7.5 - 8.5 / Temp details: / |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X06DA / Wavelength: 1.00003 Å / Beamline: X06DA / Wavelength: 1.00003 Å |
| Detector | Type: DECTRIS PILATUS 2M-F / Detector: PIXEL / Date: Oct 8, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.00003 Å / Relative weight: 1 |
| Reflection | Resolution: 0.992→108.19 Å / Num. obs: 1061996 / % possible obs: 89.1 % / Redundancy: 7 % / CC1/2: 0.997 / Rmerge(I) obs: 0.116 / Rpim(I) all: 0.047 / Rrim(I) all: 0.125 / Net I/σ(I): 9.7 |
| Reflection shell | Resolution: 0.992→1.07 Å / Rmerge(I) obs: 1.167 / Mean I/σ(I) obs: 1.6 / Num. unique obs: 53103 / CC1/2: 0.563 / Rpim(I) all: 0.476 / Rrim(I) all: 1.262 / % possible all: 58.1 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5A8W Resolution: 0.992→39.76 Å / SU ML: 0.06 / Cross valid method: THROUGHOUT / σ(F): 1.35 / Phase error: 11.99 / Stereochemistry target values: ML Details: Refinement was performed with hydrogens in riding positions
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 49.64 Å2 / Biso mean: 10.6019 Å2 / Biso min: 3.16 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 0.992→39.76 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 30
|
 Movie
Movie Controller
Controller












 PDBj
PDBj