[English] 日本語
 Yorodumi
Yorodumi- PDB-5dmj: Structure of the extracellular domain of the CD40 in complex with... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5dmj | ||||||
|---|---|---|---|---|---|---|---|
| Title | Structure of the extracellular domain of the CD40 in complex with 3H56-5 DAB | ||||||
 Components Components |
| ||||||
 Keywords Keywords | IMMUNE SYSTEM/SIGNALING PROTEIN / CELL SURFACE RECEPTOR / DOMAIN ANTIBODY / PROTEIN/PROTEIN INTERACTION / IMMUNE SYSTEM-SIGNALING PROTEIN complex | ||||||
| Function / homology |  Function and homology information Function and homology informationcellular response to erythropoietin / varicosity / B cell mediated immunity / positive regulation of interleukin-4-mediated signaling pathway / immune response-regulating cell surface receptor signaling pathway / TNF receptor superfamily (TNFSF) members mediating non-canonical NF-kB pathway / CD40 signaling pathway / CD40 receptor complex / positive regulation of isotype switching to IgG isotypes / response to cobalamin ...cellular response to erythropoietin / varicosity / B cell mediated immunity / positive regulation of interleukin-4-mediated signaling pathway / immune response-regulating cell surface receptor signaling pathway / TNF receptor superfamily (TNFSF) members mediating non-canonical NF-kB pathway / CD40 signaling pathway / CD40 receptor complex / positive regulation of isotype switching to IgG isotypes / response to cobalamin / positive regulation of protein kinase C signaling / defense response to protozoan / B cell activation / B cell proliferation / positive regulation of endothelial cell apoptotic process / positive regulation of blood vessel endothelial cell migration / cellular response to interleukin-1 / cell surface receptor signaling pathway via JAK-STAT / response to type II interferon / antigen binding / positive regulation of B cell proliferation / positive regulation of interleukin-12 production / TNFR2 non-canonical NF-kB pathway / phosphatidylinositol 3-kinase/protein kinase B signal transduction / cellular response to mechanical stimulus / platelet activation / intracellular calcium ion homeostasis / positive regulation of angiogenesis / Immunoregulatory interactions between a Lymphoid and a non-Lymphoid cell / cellular response to tumor necrosis factor / signaling receptor activity / cellular response to lipopolysaccharide / protein-containing complex assembly / defense response to virus / positive regulation of canonical NF-kappaB signal transduction / positive regulation of MAPK cascade / inflammatory response / protein domain specific binding / external side of plasma membrane / intracellular membrane-bounded organelle / neuronal cell body / ubiquitin protein ligase binding / enzyme binding / cell surface / positive regulation of transcription by RNA polymerase II / extracellular exosome / plasma membrane Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.79 Å MOLECULAR REPLACEMENT / Resolution: 2.79 Å | ||||||
 Authors Authors | Sheriff, S. | ||||||
 Citation Citation |  Journal: J.Mol.Biol. / Year: 2016 Journal: J.Mol.Biol. / Year: 2016Title: Functional Antagonism of Human CD40 Achieved by Targeting a Unique Species-Specific Epitope. Authors: Yamniuk, A.P. / Suri, A. / Krystek, S.R. / Tamura, J. / Ramamurthy, V. / Kuhn, R. / Carroll, K. / Fleener, C. / Ryseck, R. / Cheng, L. / An, Y. / Drew, P. / Grant, S. / Suchard, S.J. / ...Authors: Yamniuk, A.P. / Suri, A. / Krystek, S.R. / Tamura, J. / Ramamurthy, V. / Kuhn, R. / Carroll, K. / Fleener, C. / Ryseck, R. / Cheng, L. / An, Y. / Drew, P. / Grant, S. / Suchard, S.J. / Nadler, S.G. / Bryson, J.W. / Sheriff, S. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5dmj.cif.gz 5dmj.cif.gz | 172 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5dmj.ent.gz pdb5dmj.ent.gz | 132.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5dmj.json.gz 5dmj.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  5dmj_validation.pdf.gz 5dmj_validation.pdf.gz | 479.3 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  5dmj_full_validation.pdf.gz 5dmj_full_validation.pdf.gz | 496.6 KB | Display | |
| Data in XML |  5dmj_validation.xml.gz 5dmj_validation.xml.gz | 30.5 KB | Display | |
| Data in CIF |  5dmj_validation.cif.gz 5dmj_validation.cif.gz | 41.5 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dm/5dmj https://data.pdbj.org/pub/pdb/validation_reports/dm/5dmj ftp://data.pdbj.org/pub/pdb/validation_reports/dm/5dmj ftp://data.pdbj.org/pub/pdb/validation_reports/dm/5dmj | HTTPS FTP |
-Related structure data
| Related structure data |  5dmiC  5ihlC  1jmaS  1ncfS 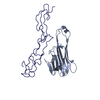 1tnrS  2aw2S  2hevS  2heyS  2uwiS  2vyrS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| 3 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 20084.449 Da / Num. of mol.: 3 / Mutation: D153, D180 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CD40, TNFRSF5 / Cell line (production host): HEK293 / Production host: Homo sapiens (human) / Gene: CD40, TNFRSF5 / Cell line (production host): HEK293 / Production host:  Homo sapiens (human) / References: UniProt: P25942 Homo sapiens (human) / References: UniProt: P25942#2: Antibody | Mass: 13272.805 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Plasmid: PDOM13 / Production host: Homo sapiens (human) / Plasmid: PDOM13 / Production host:  #3: Chemical | #4: Water | ChemComp-HOH / | Has protein modification | Y | Sequence details | THE AUTHOR STATES THAT THE PROTEIN WAS EXPRESSED WITH ASN153 AND ASN180 (AND POST-TRANSLATIONAL ...THE AUTHOR STATES THAT THE PROTEIN WAS EXPRESSED WITH ASN153 AND ASN180 (AND POST-TRANSLATIO | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.97 Å3/Da / Density % sol: 58.53 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 3.5 Details: 100mM Citric Acid, pH 3.5, 20% (w/v) PEG 4000, 200 mM potassium formate |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  CLSI CLSI  / Beamline: 08ID-1 / Wavelength: 0.9793 Å / Beamline: 08ID-1 / Wavelength: 0.9793 Å |
| Detector | Type: RAYONIX MX-225 / Detector: CCD / Date: Sep 27, 2009 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9793 Å / Relative weight: 1 |
| Reflection | Resolution: 2.79→50 Å / Num. obs: 29303 / % possible obs: 98.6 % / Observed criterion σ(I): 0 / Redundancy: 3.2 % / Biso Wilson estimate: 66.59 Å2 / Rmerge(I) obs: 0.091 / Net I/σ(I): 14.6 |
| Reflection shell | Resolution: 2.8→2.9 Å / Redundancy: 3.1 % / Rmerge(I) obs: 0.421 / Mean I/σ(I) obs: 3.4 / Rejects: 0 / % possible all: 98 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: CD40 ENSEMBLE FROM 1JMA, 2UWI, 2AW2, 1NCF, 1TNR, 2HEV, 2HEY; DAB FROM 2VYR Resolution: 2.79→46.26 Å / Cor.coef. Fo:Fc: 0.8641 / Cor.coef. Fo:Fc free: 0.8508 / SU R Cruickshank DPI: 0.734 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.698 / SU Rfree Blow DPI: 0.324 / SU Rfree Cruickshank DPI: 0.33
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 151.62 Å2 / Biso mean: 56.91 Å2 / Biso min: 6.06 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.43 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.79→46.26 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.79→2.89 Å / Total num. of bins used: 15
|
 Movie
Movie Controller
Controller









 PDBj
PDBj





