[English] 日本語
 Yorodumi
Yorodumi- PDB-4fwb: Structure of Rhodococcus rhodochrous haloalkane dehalogenase muta... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4fwb | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of Rhodococcus rhodochrous haloalkane dehalogenase mutant DhaA31 in complex with 1, 2, 3 - trichloropropane | |||||||||
 Components Components | Haloalkane dehalogenase | |||||||||
 Keywords Keywords | HYDROLASE / catalytic pentad / Structural Genomics / Enzyme Function Initiative / alpha/beta Hydrolase Fold / Halide Binding / hydrolytic dehalogenation | |||||||||
| Function / homology |  Function and homology information Function and homology informationhaloalkane dehalogenase / haloalkane dehalogenase activity / response to toxic substance / membrane Similarity search - Function | |||||||||
| Biological species |  Rhodococcus rhodochrous (bacteria) Rhodococcus rhodochrous (bacteria) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.26 Å MOLECULAR REPLACEMENT / Resolution: 1.26 Å | |||||||||
 Authors Authors | Lahoda, M. / Stsiapanava, A. / Mesters, J. / Kuta Smatanova, I. | |||||||||
 Citation Citation |  Journal: Acta Crystallogr.,Sect.D / Year: 2014 Journal: Acta Crystallogr.,Sect.D / Year: 2014Title: Crystallographic analysis of 1,2,3-trichloropropane biodegradation by the haloalkane dehalogenase DhaA31. Authors: Lahoda, M. / Mesters, J.R. / Stsiapanava, A. / Chaloupkova, R. / Kuty, M. / Damborsky, J. / Kuta Smatanova, I. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4fwb.cif.gz 4fwb.cif.gz | 140.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4fwb.ent.gz pdb4fwb.ent.gz | 107 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4fwb.json.gz 4fwb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4fwb_validation.pdf.gz 4fwb_validation.pdf.gz | 443.3 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4fwb_full_validation.pdf.gz 4fwb_full_validation.pdf.gz | 448.5 KB | Display | |
| Data in XML |  4fwb_validation.xml.gz 4fwb_validation.xml.gz | 16.9 KB | Display | |
| Data in CIF |  4fwb_validation.cif.gz 4fwb_validation.cif.gz | 26.1 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fw/4fwb https://data.pdbj.org/pub/pdb/validation_reports/fw/4fwb ftp://data.pdbj.org/pub/pdb/validation_reports/fw/4fwb ftp://data.pdbj.org/pub/pdb/validation_reports/fw/4fwb | HTTPS FTP |
-Related structure data
| Related structure data |  3rk4C  4hzgC  3fbwS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 33336.996 Da / Num. of mol.: 1 / Mutation: I135F,C176Y,V245F,L246I,Y273F Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Rhodococcus rhodochrous (bacteria) / Strain: NCIB 13064 / Gene: dhaA, DhaA31 / Plasmid: pAQN / Production host: Rhodococcus rhodochrous (bacteria) / Strain: NCIB 13064 / Gene: dhaA, DhaA31 / Plasmid: pAQN / Production host:  | ||
|---|---|---|---|
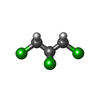
| #2: Chemical | ChemComp-3KP / | ||
| #3: Chemical | ChemComp-CL / #4: Water | ChemComp-HOH / | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.12 Å3/Da / Density % sol: 42 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 6.4 Details: 100 mM MES sodium salt, 29% (w/v) peg 4000; 1,2,3-trichloropropane was added at room temperature, pH 6.4, VAPOR DIFFUSION, SITTING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  EMBL/DESY, HAMBURG EMBL/DESY, HAMBURG  / Beamline: X12 / Wavelength: 1.033 Å / Beamline: X12 / Wavelength: 1.033 Å |
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Mar 25, 2009 |
| Radiation | Monochromator: Si (111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.033 Å / Relative weight: 1 |
| Reflection | Resolution: 1.26→10 Å / Num. all: 176338 / % possible obs: 92.4 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 2.4 % / Biso Wilson estimate: 14.6 Å2 / Rmerge(I) obs: 0.033 / Rsym value: 0.033 |
| Reflection shell | Resolution: 1.26→1.27 Å / Redundancy: 2.2 % / Rmerge(I) obs: 0.061 / Mean I/σ(I) obs: 17.6 / Rsym value: 0.061 / % possible all: 82.7 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 3fbw Resolution: 1.26→10 Å Isotropic thermal model: mixed isotropic and anisotropic thermal model Cross valid method: FREE R / σ(F): 0 / σ(I): -999 / Stereochemistry target values: ENGH AND HUBER Details: conjugate gradient least squares refinement in SHELXL 97 with anisotropic adps for all atoms except for water molecules above a certain adp cut off
| |||||||||||||||||||||||||
| Displacement parameters | Biso mean: 11.3 Å2 | |||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.12 Å / Luzzati d res low obs: 5 Å / Num. disordered residues: 26 | |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.26→10 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| LS refinement shell | Highest resolution: 1.26 Å |
 Movie
Movie Controller
Controller
















 PDBj
PDBj