[English] 日本語
 Yorodumi
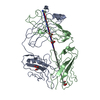
Yorodumi- PDB-1f90: FAB FRAGMENT OF MONOCLONAL ANTIBODY (LNKB-2) AGAINST HUMAN INTERL... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1f90 | ||||||
|---|---|---|---|---|---|---|---|
| Title | FAB FRAGMENT OF MONOCLONAL ANTIBODY (LNKB-2) AGAINST HUMAN INTERLEUKIN-2 IN COMPLEX WITH ANTIGENIC PEPTIDE | ||||||
 Components Components |
| ||||||
 Keywords Keywords | IMMUNE SYSTEM / monoclonal antibody / antigen-binding fragment / interleukin-2 / antigenic peptide | ||||||
| Function / homology | Immunoglobulins / Immunoglobulin-like / Sandwich / Mainly Beta Function and homology information Function and homology information | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 2.6 Å X-RAY DIFFRACTION / Resolution: 2.6 Å | ||||||
 Authors Authors | Afonin, P.V. / Fokin, A.V. / Tsigannik, I.N. / Mikhailova, I.Y. / Onoprienko, L.V. / Mikhaleva, I.I. / Ivanov, V.T. / Mareeva, T.Y. / Nesmeyanov, V.A. / Li, N. ...Afonin, P.V. / Fokin, A.V. / Tsigannik, I.N. / Mikhailova, I.Y. / Onoprienko, L.V. / Mikhaleva, I.I. / Ivanov, V.T. / Mareeva, T.Y. / Nesmeyanov, V.A. / Li, N. / Duax, W.L. / Pletnev, V.Z. | ||||||
 Citation Citation |  Journal: Protein Sci. / Year: 2001 Journal: Protein Sci. / Year: 2001Title: Crystal structure of an anti-interleukin-2 monoclonal antibody Fab complexed with an antigenic nonapeptide. Authors: Afonin, P.V. / Fokin, A.V. / Tsygannik, I.N. / Mikhailova, I.Y. / Onoprienko, L.V. / Mikhaleva, I.I. / Ivanov, V.T. / Mareeva, T.Y. / Nesmeyanov, V.A. / Li, N. / Pangborn, W.A. / Duax, W.L. / Pletnev, V.Z. #1:  Journal: BIOORG.KHIM. / Year: 2000 Journal: BIOORG.KHIM. / Year: 2000Title: THREE-DIMENSIONAL STRUCTURE OF ANTIGEN BINDING FRAGMENT OF MONOCLONAL ANTIBODY AGAINST HUMAN INTERLEUKIN-2 IN TWO CRYSTAL FORMS AT 2.2 AND 2.9 A RESOLUTION. Authors: Fokin, A.V. / Afonin, P.V. / Mikhailova, I.Y. / Tsygannik, I.N. / Mareeva, T.Y. / Nesmeyanov, V.A. / Pangborn, W. / Lee, N. / Duax, W. / Sijak, E. / Pletnev, V.Z. | ||||||
| History |
| ||||||
| Remark 999 | SEQUENCE The sequence of the protein has not been deposited in any sequence database. It was ...SEQUENCE The sequence of the protein has not been deposited in any sequence database. It was published in the Bioorganicheskaya Khimiya (Rus) (1995), V21, N6, p430-435. "Cloning cDNA Encoding Fv-Fragments of the Light and Heavy Chains of the Monoclonal Antibody" by A.I.Paskhin, T.N. Golovina, V.A. Nesmeyanov, V.G. Korobko. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1f90.cif.gz 1f90.cif.gz | 100.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1f90.ent.gz pdb1f90.ent.gz | 77.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1f90.json.gz 1f90.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/f9/1f90 https://data.pdbj.org/pub/pdb/validation_reports/f9/1f90 ftp://data.pdbj.org/pub/pdb/validation_reports/f9/1f90 ftp://data.pdbj.org/pub/pdb/validation_reports/f9/1f90 | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Antibody | Mass: 24203.908 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #2: Antibody | Mass: 23853.600 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #3: Protein/peptide | Mass: 1055.244 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: The Peptide was Chemically Synthetized in Solution |
| #4: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.39 Å3/Da / Density % sol: 48.59 % | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 300 K / Method: vapor diffusion, hanging drop / pH: 5.6 Details: PEG-4000, 2-propanol, Na-citrate , pH 5.6, VAPOR DIFFUSION, HANGING DROP, temperature 300.0K | |||||||||||||||||||||||||
| Crystal grow | *PLUS | |||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 300 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 ROTATING ANODE / Type: RIGAKU RU200 / Wavelength: 1.5418 |
| Detector | Type: RIGAKU RAXIS IIC / Detector: IMAGE PLATE / Date: Feb 5, 1999 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2.6→99 Å / Num. all: 13733 / Num. obs: 11984 / % possible obs: 83.4 % / Observed criterion σ(F): 2 / Redundancy: 4.3 % / Rmerge(I) obs: 0.148 / Net I/σ(I): 6.9 |
| Reflection shell | Resolution: 2.6→2.73 Å / Redundancy: 3.8 % / Rmerge(I) obs: 0.5825 / Num. unique all: 1850 / % possible all: 97.15 |
| Reflection | *PLUS Num. obs: 9377 / % possible obs: 99.7 % / Num. measured all: 19271 / Rmerge(I) obs: 0.112 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 2.6→99 Å / σ(F): 2 / Stereochemistry target values: Engh & Huber Details: Cross-validated maximum likelihood simulated annealing refinement (CNS package)
| |||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.6→99 Å
| |||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||
| Software | *PLUS Name: CNS / Version: 0.9 / Classification: refinement | |||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 2.6 Å / Lowest resolution: 99 Å / σ(F): 2 / % reflection Rfree: 5 % / Rfactor obs: 0.18 / Rfactor Rfree: 0.263 | |||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||
| Displacement parameters | *PLUS | |||||||||||||||||||||||||
| Refine LS restraints | *PLUS Type: c_angle_deg / Dev ideal: 1.4 |
 Movie
Movie Controller
Controller





















 PDBj
PDBj


