[English] 日本語
 Yorodumi
Yorodumi- PDB-1qv6: HORSE LIVER ALCOHOL DEHYDROGENASE HIS51GLN/LYS228ARG MUTANT COMPL... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1qv6 | ||||||
|---|---|---|---|---|---|---|---|
| Title | HORSE LIVER ALCOHOL DEHYDROGENASE HIS51GLN/LYS228ARG MUTANT COMPLEXED WITH NAD+ AND 2,4-DIFLUOROBENZYL ALCOHOL | ||||||
 Components Components | Alcohol dehydrogenase E chain | ||||||
 Keywords Keywords | OXIDOREDUCTASE / DEHYDROGENASE / ALCOHOL / NICOTINAMIDE COENZYME / 2 / 4-DIFLUOROBENZYL ALCOHOL / HIS51GLN/LYS228ARG MUTANT / HORSE LIVER | ||||||
| Function / homology |  Function and homology information Function and homology informationall-trans-retinol dehydrogenase (NAD+) activity / alcohol dehydrogenase / retinoic acid metabolic process / retinol metabolic process / zinc ion binding / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å | ||||||
 Authors Authors | Lebrun, L.A. / Park, D.-H. / Ramaswamy, S. / Plapp, B.V. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2004 Journal: Biochemistry / Year: 2004Title: Participation of histidine-51 in catalysis by horse liver alcohol dehydrogenase. Authors: LeBrun, L.A. / Park, D.-H. / Ramaswamy, S. / Plapp, B.V. #1:  Journal: Biochemistry / Year: 1994 Journal: Biochemistry / Year: 1994Title: Structures of Liver Alcohol Dehydrogenase Complexed with Nad+ and Substituted Benzyl Alcohols. Authors: Ramaswamy, S. / Eklund, H. / Plapp, B.V. #2:  Journal: Biochemistry / Year: 1999 Journal: Biochemistry / Year: 1999Title: Control of Coenzyme Binding to Horse Liver Alcohol Dehydrogenase. Authors: LeBrun, L.A. / Plapp, B.V. #3:  Journal: Biochemistry / Year: 2002 Journal: Biochemistry / Year: 2002Title: Mobility of Fluorobenzyl Alcohols Bound to Liver Alcohol Dehydrogenase as Determined by NMR and X-Ray Crystallographic Studies. Authors: Rubach, J.K. / Plapp, B.V. #4:  Journal: J.Mol.Biol. / Year: 1981 Journal: J.Mol.Biol. / Year: 1981Title: Structure of a Triclinic Ternary Complex of Horse Liver Alcohol Dehydrogenase at 2.9 A Resolution Authors: Eklund, H. / Samama, J.P. / Wallen, L. / Branden, C.I. / Akeson, A. / Jones, T.A. #5:  Journal: J.Mol.Biol. / Year: 1976 Journal: J.Mol.Biol. / Year: 1976Title: Three-Dimensional Structure of Horse Liver Alcohol Dehydrogenase at 2.4 A Resolution Authors: Eklund, H. / Nordstrom, B. / Zeppezauer, E. / Soderlund, G. / Ohlsson, I. / Boiwe, T. / Soderberg, B.O. / Tapia, O. / Branden, C.I. / Akeson, A. #6:  Journal: J.Biol.Chem. / Year: 1982 Journal: J.Biol.Chem. / Year: 1982Title: Binding of Substrate in a Ternary Complex of Horse Liver Alcohol Dehydrogenase Authors: Eklund, H. / Plapp, B.V. / Samama, J.-P. / Branden, C.-I. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1qv6.cif.gz 1qv6.cif.gz | 168.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1qv6.ent.gz pdb1qv6.ent.gz | 130.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1qv6.json.gz 1qv6.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qv/1qv6 https://data.pdbj.org/pub/pdb/validation_reports/qv/1qv6 ftp://data.pdbj.org/pub/pdb/validation_reports/qv/1qv6 ftp://data.pdbj.org/pub/pdb/validation_reports/qv/1qv6 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1qv7C  1hldS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 39871.270 Da / Num. of mol.: 2 / Fragment: Recombinant without the wild-type N-acetyl group / Mutation: H51Q and K228R Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Non-polymers , 5 types, 588 molecules 

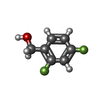






| #2: Chemical | ChemComp-ZN / #3: Chemical | #4: Chemical | #5: Chemical | ChemComp-MPD / ( | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.39 Å3/Da / Density % sol: 48.58 % | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Crystal grow | Temperature: 278 K / Method: dialysis / pH: 7 Details: 50 mM ammonium N-[tris(hydroxymethyl)methyl)]-2-aminoethanesulfonate buffer, 1 mM NAD+, 10 mM 2,4-difluorobenzyl alcohol, 2-methyl-2,4-pentanediol, pH 7.0, dialysis, temperature 278K | ||||||||||||||||||||||||||||||
| Crystal grow | *PLUS Temperature: 5 ℃ | ||||||||||||||||||||||||||||||
| Components of the solutions | *PLUS
|
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID14-4 / Wavelength: 0.9315 Å / Beamline: ID14-4 / Wavelength: 0.9315 Å |
| Detector | Type: ADSC QUANTUM 4 / Detector: CCD / Date: May 11, 1999 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9315 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→10 Å / Num. all: 63963 / Num. obs: 63963 / % possible obs: 93.3 % / Redundancy: 1.94 % / Biso Wilson estimate: 16.7 Å2 / Rmerge(I) obs: 0.063 / Net I/σ(I): 7.5 |
| Reflection shell | Resolution: 1.8→1.85 Å / Redundancy: 1.9 % / Rmerge(I) obs: 0.22 / Mean I/σ(I) obs: 3.3 / Num. unique all: 4402 / % possible all: 93.6 |
| Reflection | *PLUS Lowest resolution: 10 Å / Num. measured all: 124354 |
| Reflection shell | *PLUS % possible obs: 93.6 % |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1HLD Resolution: 1.8→10 Å / Cor.coef. Fo:Fc: 0.965 / Cor.coef. Fo:Fc free: 0.941 / SU B: 2.701 / SU ML: 0.085 / Cross valid method: THROUGHOUT / ESU R: 0.12 / ESU R Free: 0.12
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: BABINET MODEL WITH MASK | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 19.556 Å2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→10 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.8→1.845 Å / Total num. of bins used: 20 /
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Software | *PLUS Version: 5 / Classification: refinement | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement | *PLUS Highest resolution: 1.8 Å / Lowest resolution: 10 Å / Rfactor Rfree: 0.203 / Rfactor Rwork: 0.157 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | *PLUS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | *PLUS | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints | *PLUS
|
 Movie
Movie Controller
Controller























 PDBj
PDBj





