[English] 日本語
 Yorodumi
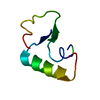
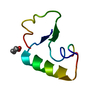
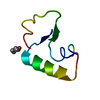
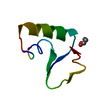
Yorodumi- PDB-1crn: WATER STRUCTURE OF A HYDROPHOBIC PROTEIN AT ATOMIC RESOLUTION. PE... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 1crn | ||||||
|---|---|---|---|---|---|---|---|
| Title | WATER STRUCTURE OF A HYDROPHOBIC PROTEIN AT ATOMIC RESOLUTION. PENTAGON RINGS OF WATER MOLECULES IN CRYSTALS OF CRAMBIN | ||||||
 Components Components | CRAMBIN | ||||||
 Keywords Keywords | PLANT PROTEIN / PLANT SEED PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology information | ||||||
| Biological species |  Crambe hispanica subsp. abyssinica (Abyssinian crambe) Crambe hispanica subsp. abyssinica (Abyssinian crambe) | ||||||
| Method |  X-RAY DIFFRACTION / Resolution: 1.5 Å X-RAY DIFFRACTION / Resolution: 1.5 Å | ||||||
 Authors Authors | Hendrickson, W.A. / Teeter, M.M. | ||||||
 Citation Citation |  Journal: Proc.Natl.Acad.Sci.Usa / Year: 1984 Journal: Proc.Natl.Acad.Sci.Usa / Year: 1984Title: Water structure of a hydrophobic protein at atomic resolution: Pentagon rings of water molecules in crystals of crambin. Authors: Teeter, M.M. #1:  Journal: Nature / Year: 1981 Journal: Nature / Year: 1981Title: Structure of the Hydrophobic Protein Crambin Determined Directly from the Anomalous Scattering of Sulphur Authors: Hendrickson, W.A. / Teeter, M.M. #2:  Journal: J.Mol.Biol. / Year: 1979 Journal: J.Mol.Biol. / Year: 1979Title: Highly Ordered Crystals of the Plant Seed Protein Crambin Authors: Teeter, M.M. / Hendrickson, W.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  1crn.cif.gz 1crn.cif.gz | 17.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb1crn.ent.gz pdb1crn.ent.gz | 10.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  1crn.json.gz 1crn.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cr/1crn https://data.pdbj.org/pub/pdb/validation_reports/cr/1crn ftp://data.pdbj.org/pub/pdb/validation_reports/cr/1crn ftp://data.pdbj.org/pub/pdb/validation_reports/cr/1crn | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein/peptide | Mass: 4738.447 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Crambe hispanica subsp. abyssinica (Abyssinian crambe) Crambe hispanica subsp. abyssinica (Abyssinian crambe)Species: Crambe hispanica / Strain: subsp. abyssinica / References: UniProt: P01542 |
|---|---|
| Compound details | THE SECONDARY STRUCTURE SPECIFICATIONS ARE THOSE DEFINED IN REFERENCE 1 ABOVE AND DEPEND ON ...THE SECONDARY STRUCTURE SPECIFICAT |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION X-RAY DIFFRACTION |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.81 Å3/Da / Density % sol: 32.16 % |
|---|---|
| Crystal grow | *PLUS Method: unknown |
| Components of the solutions | *PLUS Conc.: 60 % / Common name: ethanol |
-Data collection
| Radiation | Scattering type: x-ray |
|---|---|
| Radiation wavelength | Relative weight: 1 |
| Reflection | *PLUS Num. obs: 21045 / Observed criterion σ(F): 2 / Rmerge F obs: 0.129 |
- Processing
Processing
| Software | Name: PROLSQ / Classification: refinement | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Highest resolution: 1.5 Å | ||||||||||||
| Refinement step | Cycle: LAST / Highest resolution: 1.5 Å
| ||||||||||||
| Refinement | *PLUS Num. reflection obs: 6300 / Rfactor obs: 0.144 | ||||||||||||
| Solvent computation | *PLUS | ||||||||||||
| Displacement parameters | *PLUS |
 Movie
Movie Controller
Controller






 PDBj
PDBj
