[English] 日本語
 Yorodumi
Yorodumi- EMDB-7906: Negative stain EM map of BG505 SOSIP.644 in complex with PG9 Fab ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7906 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
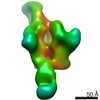
| Title | Negative stain EM map of BG505 SOSIP.644 in complex with PG9 Fab and serum from rabbit 3417 PB1 | |||||||||
 Map data Map data | Negative stain EM map of BG505 SOSIP.664 in complex with PG9 Fab and serum Fab from rabbit 3417 PB1 | |||||||||
 Sample Sample |
| |||||||||
| Biological species |   Human immunodeficiency virus Human immunodeficiency virus | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 18.45 Å | |||||||||
 Authors Authors | Turner HL / Ward AB / Nogal B | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Immunity / Year: 2018 Journal: Immunity / Year: 2018Title: Electron-Microscopy-Based Epitope Mapping Defines Specificities of Polyclonal Antibodies Elicited during HIV-1 BG505 Envelope Trimer Immunization. Authors: Matteo Bianchi / Hannah L Turner / Bartek Nogal / Christopher A Cottrell / David Oyen / Matthias Pauthner / Raiza Bastidas / Rebecca Nedellec / Laura E McCoy / Ian A Wilson / Dennis R Burton ...Authors: Matteo Bianchi / Hannah L Turner / Bartek Nogal / Christopher A Cottrell / David Oyen / Matthias Pauthner / Raiza Bastidas / Rebecca Nedellec / Laura E McCoy / Ian A Wilson / Dennis R Burton / Andrew B Ward / Lars Hangartner /   Abstract: Characterizing polyclonal antibody responses via currently available methods is inherently complex and difficult. Mapping epitopes in an immune response is typically incomplete, which creates a ...Characterizing polyclonal antibody responses via currently available methods is inherently complex and difficult. Mapping epitopes in an immune response is typically incomplete, which creates a barrier to fully understanding the humoral response to antigens and hinders rational vaccine design efforts. Here, we describe a method of characterizing polyclonal responses by using electron microscopy, and we applied this method to the immunization of rabbits with an HIV-1 envelope glycoprotein vaccine candidate, BG505 SOSIP.664. We detected known epitopes within the polyclonal sera and revealed how antibody responses evolved during the prime-boosting strategy to ultimately result in a neutralizing antibody response. We uncovered previously unidentified epitopes, including an epitope proximal to one recognized by human broadly neutralizing antibodies as well as potentially distracting non-neutralizing epitopes. Our method provides an efficient and semiquantitative map of epitopes that are targeted in a polyclonal antibody response and should be of widespread utility in vaccine and infection studies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7906.map.gz emd_7906.map.gz | 6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7906-v30.xml emd-7906-v30.xml emd-7906.xml emd-7906.xml | 11.7 KB 11.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_7906.png emd_7906.png | 30.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7906 http://ftp.pdbj.org/pub/emdb/structures/EMD-7906 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7906 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7906 | HTTPS FTP |
-Related structure data
| Related structure data |  7552C  7553C  7554C  7555C  7556C  7557C  7570C  7887C  7888C  7889C  7890C  7891C  7892C  7893C  7894C  7895C  7896C  7903C  7904C  6cjkC  6didC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_7906.map.gz / Format: CCP4 / Size: 11.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7906.map.gz / Format: CCP4 / Size: 11.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Negative stain EM map of BG505 SOSIP.664 in complex with PG9 Fab and serum Fab from rabbit 3417 PB1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
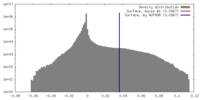
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Complex of BG505 SOSIP.664 with PG9 Fab and serum from rabbit 3417 PB1
| Entire | Name: Complex of BG505 SOSIP.664 with PG9 Fab and serum from rabbit 3417 PB1 |
|---|---|
| Components |
|
-Supramolecule #1: Complex of BG505 SOSIP.664 with PG9 Fab and serum from rabbit 3417 PB1
| Supramolecule | Name: Complex of BG505 SOSIP.664 with PG9 Fab and serum from rabbit 3417 PB1 type: complex / ID: 1 / Parent: 0 Details: Complex was purified through SEC after adding excess PG9 and serum |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus Human immunodeficiency virus |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant strain: HEK293F Homo sapiens (human) / Recombinant strain: HEK293F |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.114 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: TBS |
| Staining | Type: NEGATIVE / Material: Uranyl Formate / Details: at 2% |
| Grid | Model: Homemade / Material: COPPER / Mesh: 400 / Support film - Material: CELLULOSE ACETATE / Pretreatment - Type: GLOW DISCHARGE |
| Details | Sample in TBS, stained with UF |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI SPIRIT |
|---|---|
| Image recording | Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-46 / Number grids imaged: 1 / Number real images: 438 / Average exposure time: 0.5 sec. / Average electron dose: 25.0 e/Å2 |
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: SIDE ENTRY, EUCENTRIC |
| Experimental equipment |  Model: Tecnai Spirit / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)