[English] 日本語
 Yorodumi
Yorodumi- EMDB-7555: CryoEM map of BG505 SOSIP.664 in complex with post boost 1 serum ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-7555 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CryoEM map of BG505 SOSIP.664 in complex with post boost 1 serum from rabbit 3417 | |||||||||
 Map data Map data | BG505 SOSIP.664 in complex with serum from rabbit 3417 post boost 1. Map of antibodies bound to trimer after several rounds of 3D classification in CryoSparc. | |||||||||
 Sample Sample |
| |||||||||
| Biological species |   Human immunodeficiency virus Human immunodeficiency virus | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.7 Å | |||||||||
 Authors Authors | Turner HL / Ward AB / Nogal B | |||||||||
 Citation Citation |  Journal: Immunity / Year: 2018 Journal: Immunity / Year: 2018Title: Electron-Microscopy-Based Epitope Mapping Defines Specificities of Polyclonal Antibodies Elicited during HIV-1 BG505 Envelope Trimer Immunization. Authors: Matteo Bianchi / Hannah L Turner / Bartek Nogal / Christopher A Cottrell / David Oyen / Matthias Pauthner / Raiza Bastidas / Rebecca Nedellec / Laura E McCoy / Ian A Wilson / Dennis R Burton ...Authors: Matteo Bianchi / Hannah L Turner / Bartek Nogal / Christopher A Cottrell / David Oyen / Matthias Pauthner / Raiza Bastidas / Rebecca Nedellec / Laura E McCoy / Ian A Wilson / Dennis R Burton / Andrew B Ward / Lars Hangartner /   Abstract: Characterizing polyclonal antibody responses via currently available methods is inherently complex and difficult. Mapping epitopes in an immune response is typically incomplete, which creates a ...Characterizing polyclonal antibody responses via currently available methods is inherently complex and difficult. Mapping epitopes in an immune response is typically incomplete, which creates a barrier to fully understanding the humoral response to antigens and hinders rational vaccine design efforts. Here, we describe a method of characterizing polyclonal responses by using electron microscopy, and we applied this method to the immunization of rabbits with an HIV-1 envelope glycoprotein vaccine candidate, BG505 SOSIP.664. We detected known epitopes within the polyclonal sera and revealed how antibody responses evolved during the prime-boosting strategy to ultimately result in a neutralizing antibody response. We uncovered previously unidentified epitopes, including an epitope proximal to one recognized by human broadly neutralizing antibodies as well as potentially distracting non-neutralizing epitopes. Our method provides an efficient and semiquantitative map of epitopes that are targeted in a polyclonal antibody response and should be of widespread utility in vaccine and infection studies. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_7555.map.gz emd_7555.map.gz | 96.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-7555-v30.xml emd-7555-v30.xml emd-7555.xml emd-7555.xml | 11.3 KB 11.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_7555.png emd_7555.png | 65.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-7555 http://ftp.pdbj.org/pub/emdb/structures/EMD-7555 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7555 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-7555 | HTTPS FTP |
-Related structure data
| Related structure data |  7552C  7553C  7554C  7556C  7557C  7570C  7887C  7888C  7889C  7890C  7891C  7892C  7893C  7894C  7895C  7896C  7903C  7904C  7906C  6cjkC  6didC C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_7555.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_7555.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BG505 SOSIP.664 in complex with serum from rabbit 3417 post boost 1. Map of antibodies bound to trimer after several rounds of 3D classification in CryoSparc. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.15 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
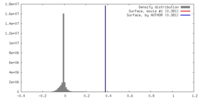
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Complex of BG505 SOSIP.664 and serum antibodies from post boost 1...
| Entire | Name: Complex of BG505 SOSIP.664 and serum antibodies from post boost 1 from rabbit 3417. Subset of particles from dataset. |
|---|---|
| Components |
|
-Supramolecule #1: Complex of BG505 SOSIP.664 and serum antibodies from post boost 1...
| Supramolecule | Name: Complex of BG505 SOSIP.664 and serum antibodies from post boost 1 from rabbit 3417. Subset of particles from dataset. type: complex / ID: 1 / Parent: 0 Details: Serum was purified through SEC after using a Protein A or G column to capture complex then cleave IgG releasing Fab and trimer. Substoichiometric occupancy and flexibility show lack of ...Details: Serum was purified through SEC after using a Protein A or G column to capture complex then cleave IgG releasing Fab and trimer. Substoichiometric occupancy and flexibility show lack of density in Fab region while trimer core is refined to higher resolution. Map shows strong density of single Fab bound to glycan hole and low densities of Fabs bound to the trimer base. |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus Human immunodeficiency virus |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant strain: HEK293F Homo sapiens (human) / Recombinant strain: HEK293F |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.44 mg/mL |
|---|---|
| Buffer | pH: 7.4 / Details: TBS |
| Grid | Model: Quantifoil R1.2/1.3 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
| Details | Sample in TBS, added 0.5uL of DDM at 0.42mM. Sample was monodisperse |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI ARCTICA |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-46 / Average exposure time: 11.5 sec. / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller











 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)