[English] 日本語
 Yorodumi
Yorodumi- EMDB-21599: Structure of human ferroportin bound to hepcidin and cobalt in li... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21599 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human ferroportin bound to hepcidin and cobalt in lipid nanodisc | |||||||||
 Map data Map data | human ferroportin bound to hepcidin and cobalt in lipid nanodisc | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | ferroportin / transporter / iron / hepcidin / TRANSLOCASE-IMMUNE SYSTEM-HORMONE complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of iron export across plasma membrane / negative regulation of intestinal absorption / spleen trabecula formation / iron ion export across plasma membrane / Defective SLC40A1 causes hemochromatosis 4 (HFE4) (duodenum) / Defective SLC40A1 causes hemochromatosis 4 (HFE4) (macrophages) / Defective CP causes aceruloplasminemia (ACERULOP) / Metal ion SLC transporters / lymphocyte homeostasis / ferrous iron transmembrane transporter activity ...negative regulation of iron export across plasma membrane / negative regulation of intestinal absorption / spleen trabecula formation / iron ion export across plasma membrane / Defective SLC40A1 causes hemochromatosis 4 (HFE4) (duodenum) / Defective SLC40A1 causes hemochromatosis 4 (HFE4) (macrophages) / Defective CP causes aceruloplasminemia (ACERULOP) / Metal ion SLC transporters / lymphocyte homeostasis / ferrous iron transmembrane transporter activity / endothelium development / iron ion transmembrane transporter activity / iron ion transmembrane transport / negative regulation of iron ion transmembrane transport / response to iron ion / transporter regulator activity / peptide hormone binding / defense response to fungus / establishment of localization in cell / Iron uptake and transport / hormone activity / multicellular organismal-level iron ion homeostasis / synaptic vesicle / positive regulation of proteasomal ubiquitin-dependent protein catabolic process / basolateral plasma membrane / killing of cells of another organism / transcription by RNA polymerase II / intracellular iron ion homeostasis / defense response to bacterium / immune response / apoptotic process / negative regulation of apoptotic process / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / extracellular space / extracellular region / nucleoplasm / metal ion binding / identical protein binding / membrane / plasma membrane / cytoplasm / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.5 Å | |||||||||
 Authors Authors | Billesboelle CB / Azumaya CM | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: Structure of hepcidin-bound ferroportin reveals iron homeostatic mechanisms. Authors: Christian B Billesbølle / Caleigh M Azumaya / Rachael C Kretsch / Alexander S Powers / Shane Gonen / Simon Schneider / Tara Arvedson / Ron O Dror / Yifan Cheng / Aashish Manglik /   Abstract: The serum level of iron in humans is tightly controlled by the action of the hormone hepcidin on the iron efflux transporter ferroportin. Hepcidin regulates iron absorption and recycling by inducing ...The serum level of iron in humans is tightly controlled by the action of the hormone hepcidin on the iron efflux transporter ferroportin. Hepcidin regulates iron absorption and recycling by inducing the internalization and degradation of ferroportin. Aberrant ferroportin activity can lead to diseases of iron overload, such as haemochromatosis, or iron limitation anaemias. Here we determine cryogenic electron microscopy structures of ferroportin in lipid nanodiscs, both in the apo state and in complex with hepcidin and the iron mimetic cobalt. These structures and accompanying molecular dynamics simulations identify two metal-binding sites within the N and C domains of ferroportin. Hepcidin binds ferroportin in an outward-open conformation and completely occludes the iron efflux pathway to inhibit transport. The carboxy terminus of hepcidin directly contacts the divalent metal in the ferroportin C domain. Hepcidin binding to ferroportin is coupled to iron binding, with an 80-fold increase in hepcidin affinity in the presence of iron. These results suggest a model for hepcidin regulation of ferroportin, in which only ferroportin molecules loaded with iron are targeted for degradation. More broadly, our structural and functional insights may enable more targeted manipulation of the hepcidin-ferroportin axis in disorders of iron homeostasis. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21599.map.gz emd_21599.map.gz | 95.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21599-v30.xml emd-21599-v30.xml emd-21599.xml emd-21599.xml | 25.3 KB 25.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_21599.png emd_21599.png | 92.2 KB | ||
| Filedesc metadata |  emd-21599.cif.gz emd-21599.cif.gz | 7.4 KB | ||
| Others |  emd_21599_half_map_1.map.gz emd_21599_half_map_1.map.gz emd_21599_half_map_2.map.gz emd_21599_half_map_2.map.gz | 37.4 MB 37.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21599 http://ftp.pdbj.org/pub/emdb/structures/EMD-21599 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21599 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21599 | HTTPS FTP |
-Related structure data
| Related structure data |  6wbvMC  6w4sC  6w4vC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21599.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21599.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | human ferroportin bound to hepcidin and cobalt in lipid nanodisc | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.834 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: #1
| File | emd_21599_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_21599_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Ferroportin-Fab45D8 complex
+Supramolecule #1: Ferroportin-Fab45D8 complex
+Supramolecule #2: Ferroportin-hepcidin complex
+Supramolecule #3: Fab45D8
+Macromolecule #1: Hepcidin
+Macromolecule #2: Solute carrier family 40 member 1
+Macromolecule #3: Fab45D8 Light Chain
+Macromolecule #4: Fab45D8 Heavy Chain
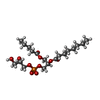
+Macromolecule #5: (1S)-2-{[{[(2S)-2,3-DIHYDROXYPROPYL]OXY}(HYDROXY)PHOSPHORYL]OXY}-...
+Macromolecule #6: COBALT (II) ION
+Macromolecule #7: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.5 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Details: unspecified |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 3 / Number real images: 5415 / Average exposure time: 6.0 sec. / Average electron dose: 66.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: -20.0 µm / Nominal defocus min: -8.0 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)