+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7egg | ||||||
|---|---|---|---|---|---|---|---|
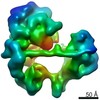
| Title | TFIID lobe B subcomplex | ||||||
 Components Components | (Transcription initiation factor TFIID subunit ...) x 7 | ||||||
 Keywords Keywords | TRANSCRIPTION / TFIID / preinitiation complex / core promoter / transcription initiation | ||||||
| Function / homology |  Function and homology information Function and homology informationSAGA complex assembly / lateral mesodermal cell differentiation / DNA-templated transcription open complex formation / allantois development / pre-snoRNP complex / transcription factor TFTC complex / SLIK (SAGA-like) complex / hepatocyte differentiation / positive regulation of response to cytokine stimulus / C2H2 zinc finger domain binding ...SAGA complex assembly / lateral mesodermal cell differentiation / DNA-templated transcription open complex formation / allantois development / pre-snoRNP complex / transcription factor TFTC complex / SLIK (SAGA-like) complex / hepatocyte differentiation / positive regulation of response to cytokine stimulus / C2H2 zinc finger domain binding / maintenance of protein location in nucleus / RNA polymerase binding / regulation of fat cell differentiation / box C/D snoRNP assembly / SAGA complex / transcription preinitiation complex / inner cell mass cell proliferation / negative regulation of intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / RNA polymerase II general transcription initiation factor activity / transcription factor TFIID complex / limb development / HIV Transcription Initiation / RNA Polymerase II HIV Promoter Escape / Transcription of the HIV genome / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance / regulation of RNA splicing / aryl hydrocarbon receptor binding / negative regulation of cell cycle / MLL1 complex / RNA polymerase II transcribes snRNA genes / positive regulation of transcription initiation by RNA polymerase II / embryonic placenta development / somitogenesis / regulation of DNA repair / RNA polymerase II preinitiation complex assembly / ovarian follicle development / positive regulation of intrinsic apoptotic signaling pathway / RNA Polymerase II Pre-transcription Events / negative regulation of proteasomal ubiquitin-dependent protein catabolic process / response to interleukin-1 / TBP-class protein binding / nuclear estrogen receptor binding / male germ cell nucleus / transcription initiation at RNA polymerase II promoter / promoter-specific chromatin binding / DNA-templated transcription initiation / G1/S transition of mitotic cell cycle / mRNA transcription by RNA polymerase II / multicellular organism growth / p53 binding / actin cytoskeleton / HATs acetylate histones / ATPase binding / Regulation of TP53 Activity through Phosphorylation / DNA-binding transcription factor binding / transcription by RNA polymerase II / transcription coactivator activity / cell differentiation / transcription cis-regulatory region binding / Ub-specific processing proteases / protein stabilization / positive regulation of apoptotic process / chromatin remodeling / protein heterodimerization activity / negative regulation of cell population proliferation / apoptotic process / DNA damage response / regulation of transcription by RNA polymerase II / regulation of DNA-templated transcription / negative regulation of apoptotic process / positive regulation of DNA-templated transcription / chromatin / perinuclear region of cytoplasm / enzyme binding / positive regulation of transcription by RNA polymerase II / protein-containing complex / DNA binding / nucleoplasm / identical protein binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 2.77 Å | ||||||
 Authors Authors | Chen, X. / Wu, Z. / Li, J. / Zhao, D. / Xu, Y. | ||||||
 Citation Citation |  Journal: Science / Year: 2021 Journal: Science / Year: 2021Title: Structural insights into preinitiation complex assembly on core promoters. Authors: Xizi Chen / Yilun Qi / Zihan Wu / Xinxin Wang / Jiabei Li / Dan Zhao / Haifeng Hou / Yan Li / Zishuo Yu / Weida Liu / Mo Wang / Yulei Ren / Ze Li / Huirong Yang / Yanhui Xu /  Abstract: Transcription factor IID (TFIID) recognizes core promoters and supports preinitiation complex (PIC) assembly for RNA polymerase II (Pol II)-mediated eukaryotic transcription. We determined the ...Transcription factor IID (TFIID) recognizes core promoters and supports preinitiation complex (PIC) assembly for RNA polymerase II (Pol II)-mediated eukaryotic transcription. We determined the structures of human TFIID-based PIC in three stepwise assembly states and revealed two-track PIC assembly: stepwise promoter deposition to Pol II and extensive modular reorganization on track I (on TATA-TFIID-binding element promoters) versus direct promoter deposition on track II (on TATA-only and TATA-less promoters). The two tracks converge at an ~50-subunit holo PIC in identical conformation, whereby TFIID stabilizes PIC organization and supports loading of cyclin-dependent kinase (CDK)-activating kinase (CAK) onto Pol II and CAK-mediated phosphorylation of the Pol II carboxyl-terminal domain. Unexpectedly, TBP of TFIID similarly bends TATA box and TATA-less promoters in PIC. Our study provides structural visualization of stepwise PIC assembly on highly diversified promoters. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7egg.cif.gz 7egg.cif.gz | 276.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7egg.ent.gz pdb7egg.ent.gz | 193.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7egg.json.gz 7egg.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/eg/7egg https://data.pdbj.org/pub/pdb/validation_reports/eg/7egg ftp://data.pdbj.org/pub/pdb/validation_reports/eg/7egg ftp://data.pdbj.org/pub/pdb/validation_reports/eg/7egg | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  31116MC  7edxC  7eg7C  7eg8C  7eg9C  7egaC  7egbC  7egcC  7egdC  7egeC  7egfC  7eghC  7egiC  7egjC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Transcription initiation factor TFIID subunit ... , 7 types, 7 molecules DEFHIJL
| #1: Protein | Mass: 110221.883 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TAF4, TAF2C, TAF2C1, TAF4A, TAFII130, TAFII135 / Production host: Homo sapiens (human) / Gene: TAF4, TAF2C, TAF2C1, TAF4A, TAFII130, TAFII135 / Production host:  Homo sapiens (human) / References: UniProt: O00268 Homo sapiens (human) / References: UniProt: O00268 |
|---|---|
| #2: Protein | Mass: 86932.109 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TAF5, TAF2D / Production host: Homo sapiens (human) / Gene: TAF5, TAF2D / Production host:  Homo sapiens (human) / References: UniProt: Q15542 Homo sapiens (human) / References: UniProt: Q15542 |
| #3: Protein | Mass: 72749.297 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TAF6, TAF2E, TAFII70 / Production host: Homo sapiens (human) / Gene: TAF6, TAF2E, TAFII70 / Production host:  Homo sapiens (human) / References: UniProt: P49848 Homo sapiens (human) / References: UniProt: P49848 |
| #4: Protein | Mass: 34304.359 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TAF8, TAFII43, TBN / Production host: Homo sapiens (human) / Gene: TAF8, TAFII43, TBN / Production host:  Homo sapiens (human) / References: UniProt: Q7Z7C8 Homo sapiens (human) / References: UniProt: Q7Z7C8 |
| #5: Protein | Mass: 29006.838 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TAF9, TAF2G, TAFII31 / Production host: Homo sapiens (human) / Gene: TAF9, TAF2G, TAFII31 / Production host:  Homo sapiens (human) / References: UniProt: Q16594 Homo sapiens (human) / References: UniProt: Q16594 |
| #6: Protein | Mass: 21731.248 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TAF10, TAF2A, TAF2H, TAFII30 / Production host: Homo sapiens (human) / Gene: TAF10, TAF2A, TAF2H, TAFII30 / Production host:  Homo sapiens (human) / References: UniProt: Q12962 Homo sapiens (human) / References: UniProt: Q12962 |
| #7: Protein | Mass: 17948.467 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: TAF12, TAF15, TAF2J, TAFII20 / Production host: Homo sapiens (human) / Gene: TAF12, TAF15, TAF2J, TAFII20 / Production host:  Homo sapiens (human) / References: UniProt: Q16514 Homo sapiens (human) / References: UniProt: Q16514 |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: TFIID lobe B subcomplex / Type: COMPLEX / Entity ID: all / Source: RECOMBINANT |
|---|---|
| Molecular weight | Units: KILODALTONS/NANOMETER / Experimental value: NO |
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.9 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Specimen support | Grid material: GOLD |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Cs: 2.7 mm |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Electron dose: 63 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
| EM imaging optics | Energyfilter slit width: 20 eV |
| Image scans | Movie frames/image: 32 |
- Processing
Processing
| CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
|---|---|
| 3D reconstruction | Resolution: 2.77 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 84427 / Symmetry type: POINT |
| Atomic model building | Protocol: AB INITIO MODEL / Space: REAL |
 Movie
Movie Controller
Controller








































 PDBj
PDBj




