+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-31118 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
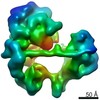
| Title | TFIID in rearranged conformation | |||||||||
 Map data Map data | TFIID in rearranged conformation | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | TFIID / preinitiation complex / core promoter / transcription initiation / TRANSCRIPTION | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of MHC class I biosynthetic process / spermine transport / SAGA complex assembly / lateral mesodermal cell differentiation / DNA-templated transcription open complex formation / allantois development / pre-snoRNP complex / positive regulation of androgen receptor signaling pathway / TFIIH-class transcription factor complex binding / negative regulation of protein autoubiquitination ...negative regulation of MHC class I biosynthetic process / spermine transport / SAGA complex assembly / lateral mesodermal cell differentiation / DNA-templated transcription open complex formation / allantois development / pre-snoRNP complex / positive regulation of androgen receptor signaling pathway / TFIIH-class transcription factor complex binding / negative regulation of protein autoubiquitination / negative regulation of MHC class II biosynthetic process / transcription factor TFTC complex / RNA polymerase I general transcription initiation factor activity / regulation of cell cycle G1/S phase transition / RNA polymerase transcription factor SL1 complex / histone H4K16ac reader activity / SLIK (SAGA-like) complex / RNA polymerase III general transcription initiation factor activity / RNA polymerase I core promoter sequence-specific DNA binding / hepatocyte differentiation / positive regulation of response to cytokine stimulus / RNA Polymerase III Transcription Initiation From Type 1 Promoter / RNA Polymerase III Transcription Initiation From Type 2 Promoter / RNA Polymerase III Transcription Initiation From Type 3 Promoter / transcription factor TFIIA complex / maintenance of protein location in nucleus / C2H2 zinc finger domain binding / female germ cell nucleus / RNA Polymerase III Abortive And Retractive Initiation / histone H3K4me3 reader activity / male pronucleus / host-mediated activation of viral transcription / female pronucleus / RNA polymerase II general transcription initiation factor binding / nuclear vitamin D receptor binding / RNA polymerase binding / regulation of fat cell differentiation / nuclear thyroid hormone receptor binding / limb development / box C/D snoRNP assembly / SAGA complex / transcription preinitiation complex / RNA Polymerase I Transcription Termination / inner cell mass cell proliferation / RNA polymerase II general transcription initiation factor activity / negative regulation of intrinsic apoptotic signaling pathway in response to DNA damage by p53 class mediator / transcription factor TFIID complex / histone acetyltransferase binding / HIV Transcription Initiation / RNA Polymerase II HIV Promoter Escape / Transcription of the HIV genome / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance / midbrain development / cellular response to ATP / regulation of RNA splicing / negative regulation of signal transduction by p53 class mediator / negative regulation of cell cycle / aryl hydrocarbon receptor binding / transcription initiation at RNA polymerase I promoter / ubiquitin conjugating enzyme activity / TFIIB-class transcription factor binding / P-TEFb complex binding / RNA Polymerase I Transcription Initiation / MLL1 complex / transcription by RNA polymerase III / RNA polymerase II transcribes snRNA genes / negative regulation of ubiquitin-dependent protein catabolic process / positive regulation of transcription initiation by RNA polymerase II / embryonic placenta development / somitogenesis / histone acetyltransferase activity / core promoter sequence-specific DNA binding / RNA polymerase II core promoter sequence-specific DNA binding / regulation of DNA repair / negative regulation of protein kinase activity / RNA polymerase II preinitiation complex assembly / histone acetyltransferase / transcription regulator inhibitor activity / ovarian follicle development / estrogen receptor signaling pathway / positive regulation of intrinsic apoptotic signaling pathway / RNA Polymerase II Pre-transcription Events / negative regulation of proteasomal ubiquitin-dependent protein catabolic process / response to interleukin-1 / TBP-class protein binding / regulation of signal transduction by p53 class mediator / nuclear estrogen receptor binding / nuclear receptor binding / SIRT1 negatively regulates rRNA expression / male germ cell nucleus / transcription initiation at RNA polymerase II promoter / promoter-specific chromatin binding / RNA Polymerase I Promoter Escape / DNA-templated transcription initiation / mRNA transcription by RNA polymerase II / G1/S transition of mitotic cell cycle / euchromatin Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 9.82 Å | |||||||||
 Authors Authors | Chen X / Wu Z | |||||||||
 Citation Citation |  Journal: Science / Year: 2021 Journal: Science / Year: 2021Title: Structural insights into preinitiation complex assembly on core promoters. Authors: Xizi Chen / Yilun Qi / Zihan Wu / Xinxin Wang / Jiabei Li / Dan Zhao / Haifeng Hou / Yan Li / Zishuo Yu / Weida Liu / Mo Wang / Yulei Ren / Ze Li / Huirong Yang / Yanhui Xu /  Abstract: Transcription factor IID (TFIID) recognizes core promoters and supports preinitiation complex (PIC) assembly for RNA polymerase II (Pol II)-mediated eukaryotic transcription. We determined the ...Transcription factor IID (TFIID) recognizes core promoters and supports preinitiation complex (PIC) assembly for RNA polymerase II (Pol II)-mediated eukaryotic transcription. We determined the structures of human TFIID-based PIC in three stepwise assembly states and revealed two-track PIC assembly: stepwise promoter deposition to Pol II and extensive modular reorganization on track I (on TATA-TFIID-binding element promoters) versus direct promoter deposition on track II (on TATA-only and TATA-less promoters). The two tracks converge at an ~50-subunit holo PIC in identical conformation, whereby TFIID stabilizes PIC organization and supports loading of cyclin-dependent kinase (CDK)-activating kinase (CAK) onto Pol II and CAK-mediated phosphorylation of the Pol II carboxyl-terminal domain. Unexpectedly, TBP of TFIID similarly bends TATA box and TATA-less promoters in PIC. Our study provides structural visualization of stepwise PIC assembly on highly diversified promoters. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_31118.map.gz emd_31118.map.gz | 8.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-31118-v30.xml emd-31118-v30.xml emd-31118.xml emd-31118.xml | 34.5 KB 34.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_31118.png emd_31118.png | 90.3 KB | ||
| Filedesc metadata |  emd-31118.cif.gz emd-31118.cif.gz | 11.7 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-31118 http://ftp.pdbj.org/pub/emdb/structures/EMD-31118 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31118 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-31118 | HTTPS FTP |
-Validation report
| Summary document |  emd_31118_validation.pdf.gz emd_31118_validation.pdf.gz | 341.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_31118_full_validation.pdf.gz emd_31118_full_validation.pdf.gz | 340.7 KB | Display | |
| Data in XML |  emd_31118_validation.xml.gz emd_31118_validation.xml.gz | 6.6 KB | Display | |
| Data in CIF |  emd_31118_validation.cif.gz emd_31118_validation.cif.gz | 7.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31118 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31118 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31118 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-31118 | HTTPS FTP |
-Related structure data
| Related structure data |  7egiMC  7edxC  7eg7C  7eg8C  7eg9C  7egaC  7egbC  7egcC  7egdC  7egeC  7egfC  7eggC  7eghC  7egjC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_31118.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_31118.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | TFIID in rearranged conformation | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.35 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : TFIID in rearranged conformation
+Supramolecule #1: TFIID in rearranged conformation
+Macromolecule #1: Transcription initiation factor TFIID subunit 1
+Macromolecule #2: Transcription initiation factor TFIID subunit 2
+Macromolecule #3: Transcription initiation factor TFIID subunit 4
+Macromolecule #4: Transcription initiation factor TFIID subunit 5
+Macromolecule #5: Transcription initiation factor TFIID subunit 6
+Macromolecule #6: Transcription initiation factor TFIID subunit 7
+Macromolecule #7: Transcription initiation factor TFIID subunit 8
+Macromolecule #8: Transcription initiation factor TFIID subunit 9
+Macromolecule #9: Transcription initiation factor TFIID subunit 10
+Macromolecule #10: Transcription initiation factor TFIID subunit 12
+Macromolecule #11: Transcription initiation factor IIA subunit 2
+Macromolecule #12: TATA-box-binding protein
+Macromolecule #13: Transcription initiation factor IIA subunit 1
+Macromolecule #14: Transcription initiation factor TFIID subunit 3
+Macromolecule #15: Transcription initiation factor TFIID subunit 11
+Macromolecule #16: Transcription initiation factor TFIID subunit 13
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.9 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Support film - Material: CARBON / Support film - topology: CONTINUOUS |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: SUPER-RESOLUTION / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 9.82 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 64496 |
| Initial angle assignment | Type: OTHER |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
-Atomic model buiding 1
| Refinement | Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-7egi: |
 Movie
Movie Controller
Controller












































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















