[English] 日本語
 Yorodumi
Yorodumi- PDB-6uoc: Crystal structure of Danio rerio histone deacetylase 6 catalytic ... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6uoc | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Danio rerio histone deacetylase 6 catalytic domain 1 (CD1) K330L mutant complexed with Givinostat | ||||||
 Components Components | Histone deacetylase 6 | ||||||
 Keywords Keywords | Hydrolase/Hydrolase Inhibitor / Histone Deacetylase / Hydrolase-Hydrolase Inhibitor complex | ||||||
| Function / homology |  Function and homology information Function and homology informationAggrephagy / negative regulation of cellular component organization / positive regulation of cellular component organization / regulation of biological quality / deacetylase activity / tubulin deacetylase activity / mitochondrion localization / definitive hemopoiesis / regulation of microtubule-based process / protein lysine deacetylase activity ...Aggrephagy / negative regulation of cellular component organization / positive regulation of cellular component organization / regulation of biological quality / deacetylase activity / tubulin deacetylase activity / mitochondrion localization / definitive hemopoiesis / regulation of microtubule-based process / protein lysine deacetylase activity / potassium ion binding / response to stress / hematopoietic progenitor cell differentiation / swimming behavior / transferase activity / actin binding / chromatin organization / angiogenesis / perikaryon / axon / dendrite / centrosome / zinc ion binding / nucleus / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.40000794136 Å MOLECULAR REPLACEMENT / Resolution: 1.40000794136 Å | ||||||
 Authors Authors | Osko, J.D. / Christianson, D.W. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2019 Journal: Biochemistry / Year: 2019Title: Structural Basis of Catalysis and Inhibition of HDAC6 CD1, the Enigmatic Catalytic Domain of Histone Deacetylase 6. Authors: Osko, J.D. / Christianson, D.W. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6uoc.cif.gz 6uoc.cif.gz | 123.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6uoc.ent.gz pdb6uoc.ent.gz | 73.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6uoc.json.gz 6uoc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/uo/6uoc https://data.pdbj.org/pub/pdb/validation_reports/uo/6uoc ftp://data.pdbj.org/pub/pdb/validation_reports/uo/6uoc ftp://data.pdbj.org/pub/pdb/validation_reports/uo/6uoc | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6uo2C  6uo3C  6uo4C  6uo5C  6uo7C  6uobC  5eefS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 40278.570 Da / Num. of mol.: 1 / Mutation: K330L Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|
-Non-polymers , 5 types, 439 molecules 


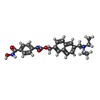





| #2: Chemical | | #3: Chemical | ChemComp-ZN / | #4: Chemical | #5: Chemical | ChemComp-QCM / | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.53 Å3/Da / Density % sol: 51.31 % / Description: Thin/Thick Plate-Like Shape |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop Details: 10 mg/ml HDAC6 CD1 2 mM Inhibitor 0.2 M magnesium formate dihydrate 20% PEG 3350 1:1 ratio protein to precipitant |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 24-ID-E / Wavelength: 0.98 Å / Beamline: 24-ID-E / Wavelength: 0.98 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Jul 8, 2019 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.98 Å / Relative weight: 1 |
| Reflection | Resolution: 1.4→42.9476472842 Å / Num. obs: 80865 / % possible obs: 99.7 % / Redundancy: 6.5 % / Biso Wilson estimate: 11.016022672 Å2 / CC1/2: 0.995 / Rmerge(I) obs: 0.094 / Rpim(I) all: 0.04 / Net I/σ(I): 12.4 |
| Reflection shell | Resolution: 1.4→1.45 Å / Redundancy: 6.7 % / Rmerge(I) obs: 0.359 / Mean I/σ(I) obs: 5.6 / Num. unique obs: 7985 / CC1/2: 0.952 / Rpim(I) all: 0.15 / % possible all: 99.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5EEF Resolution: 1.40000794136→42.9476472842 Å / SU ML: 0.095673127259 / Cross valid method: FREE R-VALUE / σ(F): 1.33792355939 / Phase error: 15.2008364571
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 15.2124829674 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.40000794136→42.9476472842 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj



