[English] 日本語
 Yorodumi
Yorodumi- PDB-6ilj: Cryo-EM structure of Echovirus 6 complexed with its attachment re... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6ilj | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of Echovirus 6 complexed with its attachment receptor CD55 at PH 5.5 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Components Components |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Keywords Keywords | VIRUS / Echovirus 6 / CD55 / Cryo-EM / virus-receptor complex | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of lipopolysaccharide-mediated signaling pathway / negative regulation of complement activation / regulation of complement-dependent cytotoxicity / regulation of complement activation / respiratory burst / positive regulation of CD4-positive, alpha-beta T cell activation / positive regulation of CD4-positive, alpha-beta T cell proliferation / Class B/2 (Secretin family receptors) / ficolin-1-rich granule membrane / complement activation, classical pathway ...regulation of lipopolysaccharide-mediated signaling pathway / negative regulation of complement activation / regulation of complement-dependent cytotoxicity / regulation of complement activation / respiratory burst / positive regulation of CD4-positive, alpha-beta T cell activation / positive regulation of CD4-positive, alpha-beta T cell proliferation / Class B/2 (Secretin family receptors) / ficolin-1-rich granule membrane / complement activation, classical pathway / transport vesicle / side of membrane / COPI-mediated anterograde transport / endoplasmic reticulum-Golgi intermediate compartment membrane / secretory granule membrane / Regulation of Complement cascade / positive regulation of T cell cytokine production / positive regulation of cytosolic calcium ion concentration / virus receptor activity / membrane raft / Golgi membrane / innate immune response / Neutrophil degranulation / lipid binding / cell surface / extracellular exosome / extracellular region / plasma membrane Similarity search - Function | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) Echovirus E6 Echovirus E6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.6 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Authors Authors | Gao, G.F. / Liu, S. / Zhao, X. / Peng, R. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Funding support |  China, 1items China, 1items
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Citation Citation |  Journal: Cell / Year: 2019 Journal: Cell / Year: 2019Title: Human Neonatal Fc Receptor Is the Cellular Uncoating Receptor for Enterovirus B. Authors: Xin Zhao / Guigen Zhang / Sheng Liu / Xiangpeng Chen / Ruchao Peng / Lianpan Dai / Xiao Qu / Shihua Li / Hao Song / Zhengrong Gao / Pengfei Yuan / Zhiheng Liu / Changyao Li / Zifang Shang / ...Authors: Xin Zhao / Guigen Zhang / Sheng Liu / Xiangpeng Chen / Ruchao Peng / Lianpan Dai / Xiao Qu / Shihua Li / Hao Song / Zhengrong Gao / Pengfei Yuan / Zhiheng Liu / Changyao Li / Zifang Shang / Yan Li / Meifan Zhang / Jianxun Qi / Han Wang / Ning Du / Yan Wu / Yuhai Bi / Shan Gao / Yi Shi / Jinghua Yan / Yong Zhang / Zhengde Xie / Wensheng Wei / George F Gao /  Abstract: Enterovirus B (EV-B), a major proportion of the genus Enterovirus in the family Picornaviridae, is the causative agent of severe human infectious diseases. Although cellular receptors for ...Enterovirus B (EV-B), a major proportion of the genus Enterovirus in the family Picornaviridae, is the causative agent of severe human infectious diseases. Although cellular receptors for coxsackievirus B in EV-B have been identified, receptors mediating virus entry, especially the uncoating process of echovirus and other EV-B remain obscure. Here, we found that human neonatal Fc receptor (FcRn) is the uncoating receptor for major EV-B. FcRn binds to the virus particles in the "canyon" through its FCGRT subunit. By obtaining multiple cryo-electron microscopy structures at different stages of virus entry at atomic or near-atomic resolution, we deciphered the underlying mechanisms of enterovirus attachment and uncoating. These structures revealed that different from the attachment receptor CD55, binding of FcRn to the virions induces efficient release of "pocket factor" under acidic conditions and initiates the conformational changes in viral particle, providing a structural basis for understanding the mechanisms of enterovirus entry. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6ilj.cif.gz 6ilj.cif.gz | 197 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6ilj.ent.gz pdb6ilj.ent.gz | 153 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6ilj.json.gz 6ilj.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/il/6ilj https://data.pdbj.org/pub/pdb/validation_reports/il/6ilj ftp://data.pdbj.org/pub/pdb/validation_reports/il/6ilj ftp://data.pdbj.org/pub/pdb/validation_reports/il/6ilj | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  9684MC  9685C  9686C  9687C  9688C  9689C  9690C  6ilkC  6illC  6ilmC  6ilnC  6iloC  6ilpC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 | x 60
|
| 2 |
|
| 3 | x 5
|
| 4 | x 6
|
| 5 | 
|
| Symmetry | Point symmetry: (Schoenflies symbol: I (icosahedral)) |
- Components
Components
-Capsid protein ... , 4 types, 4 molecules ABCD
| #1: Protein | Mass: 31707.332 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Echovirus E6 Echovirus E6 |
|---|---|
| #2: Protein | Mass: 28064.520 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Echovirus E6 Echovirus E6 |
| #3: Protein | Mass: 26378.936 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Echovirus E6 Echovirus E6 |
| #4: Protein | Mass: 7338.014 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  Echovirus E6 Echovirus E6 |
-Protein / Non-polymers , 2 types, 2 molecules E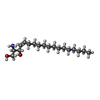

| #5: Protein | Mass: 21307.959 Da / Num. of mol.: 1 / Fragment: UNP residues 94-285 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CD55, CR, DAF / Cell (production host): 293T / Production host: Homo sapiens (human) / Gene: CD55, CR, DAF / Cell (production host): 293T / Production host:  Homo sapiens (human) / References: UniProt: P08174 Homo sapiens (human) / References: UniProt: P08174 |
|---|---|
| #6: Chemical | ChemComp-SPH / |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Cryo-EM structure of Echovirus 6 complexed with its attachment receptor CD55 at PH 5.5 Type: COMPLEX / Entity ID: #1-#5 / Source: MULTIPLE SOURCES |
|---|---|
| Molecular weight | Units: MEGADALTONS / Experimental value: NO |
| Source (natural) | Organism:  Echovirus E6 Echovirus E6 |
| Natural host | Organism: Homo sapiens |
| Buffer solution | pH: 5.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD |
| Image recording | Electron dose: 40 e/Å2 / Detector mode: SUPER-RESOLUTION / Film or detector model: GATAN K2 QUANTUM (4k x 4k) |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.11.1_2575: / Classification: refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software | Name: PHENIX / Category: model refinement | ||||||||||||||||||||||||
| CTF correction | Type: NONE | ||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 11494 / Symmetry type: POINT | ||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj






