[English] 日本語
 Yorodumi
Yorodumi- PDB-6gks: X-ray structure determined from recombinant Charcot-Leyden crystal -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6gks | ||||||
|---|---|---|---|---|---|---|---|
| Title | X-ray structure determined from recombinant Charcot-Leyden crystal | ||||||
 Components Components | Galectin-10 | ||||||
 Keywords Keywords | SUGAR BINDING PROTEIN / recombinant / human / galectin-10 | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of activated T cell proliferation / regulation of T cell cytokine production / T cell apoptotic process / regulation of T cell anergy / : / carbohydrate binding / identical protein binding / cytosol Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.38 Å MOLECULAR REPLACEMENT / Resolution: 1.38 Å | ||||||
 Authors Authors | Verstraete, K. / Verschueren, K. / Savvides, S.N. | ||||||
 Citation Citation |  Journal: Science / Year: 2019 Journal: Science / Year: 2019Title: Protein crystallization promotes type 2 immunity and is reversible by antibody treatment. Authors: Emma K Persson / Kenneth Verstraete / Ines Heyndrickx / Elien Gevaert / Helena Aegerter / Jean-Michel Percier / Kim Deswarte / Koen H G Verschueren / Ann Dansercoer / Delphine Gras / Pascal ...Authors: Emma K Persson / Kenneth Verstraete / Ines Heyndrickx / Elien Gevaert / Helena Aegerter / Jean-Michel Percier / Kim Deswarte / Koen H G Verschueren / Ann Dansercoer / Delphine Gras / Pascal Chanez / Claus Bachert / Amanda Gonçalves / Hanne Van Gorp / Hans De Haard / Christophe Blanchetot / Michael Saunders / Hamida Hammad / Savvas N Savvides / Bart N Lambrecht /     Abstract: Although spontaneous protein crystallization is a rare event in vivo, Charcot-Leyden crystals (CLCs) consisting of galectin-10 (Gal10) protein are frequently observed in eosinophilic diseases, such ...Although spontaneous protein crystallization is a rare event in vivo, Charcot-Leyden crystals (CLCs) consisting of galectin-10 (Gal10) protein are frequently observed in eosinophilic diseases, such as asthma. We found that CLCs derived from patients showed crystal packing and Gal10 structure identical to those of Gal10 crystals grown in vitro. When administered to the airways, crystalline Gal10 stimulated innate and adaptive immunity and acted as a type 2 adjuvant. By contrast, a soluble Gal10 mutein was inert. Antibodies directed against key epitopes of the CLC crystallization interface dissolved preexisting CLCs in patient-derived mucus within hours and reversed crystal-driven inflammation, goblet-cell metaplasia, immunoglobulin E (IgE) synthesis, and bronchial hyperreactivity (BHR) in a humanized mouse model of asthma. Thus, protein crystals may promote hallmark features of asthma and are targetable by crystal-dissolving antibodies. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6gks.cif.gz 6gks.cif.gz | 84.5 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6gks.ent.gz pdb6gks.ent.gz | 63.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6gks.json.gz 6gks.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gk/6gks https://data.pdbj.org/pub/pdb/validation_reports/gk/6gks ftp://data.pdbj.org/pub/pdb/validation_reports/gk/6gks ftp://data.pdbj.org/pub/pdb/validation_reports/gk/6gks | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6gkqC  6gktC  6gkuC  6glwC  6glxC  6qrnC  1lclS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 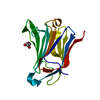
| |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||
| Unit cell |
| |||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 16667.031 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: Recombinant human galectin-10 was produced with an N-terminal cleavable His-tag (MASTTHHHHHHDTDIPTTGGGSRPDDDDKENLYFQG). Upon TEV-mediated removal of the His-tag recombinant human galectin-10 ...Details: Recombinant human galectin-10 was produced with an N-terminal cleavable His-tag (MASTTHHHHHHDTDIPTTGGGSRPDDDDKENLYFQG). Upon TEV-mediated removal of the His-tag recombinant human galectin-10 autocrystallized in PBS buffer. Source: (gene. exp.)  Homo sapiens (human) / Gene: CLC, LGALS10, LGALS10A / Plasmid: pET28 / Production host: Homo sapiens (human) / Gene: CLC, LGALS10, LGALS10A / Plasmid: pET28 / Production host:  |
|---|---|
| #2: Chemical | ChemComp-GOL / |
| #3: Water | ChemComp-HOH / |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.68 Å3/Da / Density % sol: 54.07 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: batch mode / pH: 7.4 Details: Upon removal of the N-terminal His-tag by TEV protease, at a protease:target protein ratio of 1:100 (w/w), recombinant galectin-10 autocrystallized in PBS buffer. Temp details: Room temperature |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  PETRA III, EMBL c/o DESY PETRA III, EMBL c/o DESY  / Beamline: P14 (MX2) / Wavelength: 0.9763 Å / Beamline: P14 (MX2) / Wavelength: 0.9763 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Sep 22, 2015 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9763 Å / Relative weight: 1 |
| Reflection | Resolution: 1.34→50 Å / Num. obs: 39313 / % possible obs: 92.4 % / Redundancy: 17.9 % / Biso Wilson estimate: 10.1 Å2 / CC1/2: 0.99 / Rrim(I) all: 0.076 / Net I/σ(I): 25.64 |
| Reflection shell | Resolution: 1.34→1.42 Å / Redundancy: 14.6 % / Mean I/σ(I) obs: 5.8 / Num. unique obs: 4579 / Rrim(I) all: 0.72 / % possible all: 68.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 1LCL Resolution: 1.38→43.105 Å / SU ML: 0.11 / Cross valid method: FREE R-VALUE / σ(F): 1.35 / Phase error: 15.38
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.38→43.105 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: -22.6572 Å / Origin y: 17.4215 Å / Origin z: 8.3791 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group | Selection details: all |
 Movie
Movie Controller
Controller













 PDBj
PDBj


