+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5y5e | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of phospholipase A2 with inhibitor | ||||||
 Components Components | Group IIE secretory phospholipase A2 | ||||||
 Keywords Keywords | HYDROLASE/INHIBITOR / mutant / HYDROLASE-INHIBITOR complex | ||||||
| Function / homology |  Function and homology information Function and homology informationAcyl chain remodelling of PG / Acyl chain remodelling of PC / Acyl chain remodelling of PI / Acyl chain remodelling of PS / Acyl chain remodelling of PE / Synthesis of PA / phosphatidylglycerol metabolic process / A2-type glycerophospholipase activity / phosphatidylcholine metabolic process / phospholipase A2 ...Acyl chain remodelling of PG / Acyl chain remodelling of PC / Acyl chain remodelling of PI / Acyl chain remodelling of PS / Acyl chain remodelling of PE / Synthesis of PA / phosphatidylglycerol metabolic process / A2-type glycerophospholipase activity / phosphatidylcholine metabolic process / phospholipase A2 / low-density lipoprotein particle remodeling / : / arachidonate secretion / lipid catabolic process / phospholipid metabolic process / phospholipid binding / inflammatory response / calcium ion binding / extracellular region / cytoplasm Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å | ||||||
 Authors Authors | Hou, S. / Xu, J. / Xu, T. / Liu, J. | ||||||
 Citation Citation |  Journal: Sci Rep / Year: 2017 Journal: Sci Rep / Year: 2017Title: Structural basis for functional selectivity and ligand recognition revealed by crystal structures of human secreted phospholipase A2group IIE Authors: Hou, S. / Xu, T. / Xu, J. / Qu, L. / Xu, Y. / Chen, L. / Liu, J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5y5e.cif.gz 5y5e.cif.gz | 46.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5y5e.ent.gz pdb5y5e.ent.gz | 30.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5y5e.json.gz 5y5e.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/y5/5y5e https://data.pdbj.org/pub/pdb/validation_reports/y5/5y5e ftp://data.pdbj.org/pub/pdb/validation_reports/y5/5y5e ftp://data.pdbj.org/pub/pdb/validation_reports/y5/5y5e | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5wzmSC  5wzoC  5wzsC  5wztC  5wzuC  5wzvC  5wzwC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 13929.085 Da / Num. of mol.: 1 / Fragment: UNP residues 20-142 / Mutation: N21G Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: PLA2G2E / Plasmid: pGAPZaA / Production host: Homo sapiens (human) / Gene: PLA2G2E / Plasmid: pGAPZaA / Production host:  Komagataella pastoris (fungus) / Strain (production host): X33 / References: UniProt: Q9NZK7, phospholipase A2 Komagataella pastoris (fungus) / Strain (production host): X33 / References: UniProt: Q9NZK7, phospholipase A2 |
|---|
-Non-polymers , 7 types, 154 molecules 











| #2: Chemical | ChemComp-GOL / | ||||||||
|---|---|---|---|---|---|---|---|---|---|
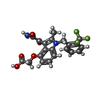
| #3: Chemical | ChemComp-7W3 / | ||||||||
| #4: Chemical | ChemComp-CL / #5: Chemical | ChemComp-CA / | #6: Chemical | ChemComp-B3P / | #7: Chemical | ChemComp-NA / | #8: Water | ChemComp-HOH / | |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.44 Å3/Da / Density % sol: 64.24 % / Mosaicity: 0.71 ° |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion / Details: 2.2M Sodium chloride, 0.1M BIS-TRIS propane pH 7.0 |
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: OXFORD DIFFRACTION ENHANCE ULTRA / Wavelength: 1.5418 Å ROTATING ANODE / Type: OXFORD DIFFRACTION ENHANCE ULTRA / Wavelength: 1.5418 Å | ||||||||||||||||||||||||
| Detector | Type: OXFORD RUBY CCD / Detector: CCD / Date: Jun 9, 2015 | ||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 | ||||||||||||||||||||||||
| Reflection | Resolution: 1.8→18.78 Å / Num. obs: 18109 / % possible obs: 99.9 % / Redundancy: 9.5 % / CC1/2: 0.999 / Rmerge(I) obs: 0.06 / Rpim(I) all: 0.02 / Rrim(I) all: 0.064 / Net I/σ(I): 24.8 | ||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5WZM Resolution: 1.8→18.78 Å / Cor.coef. Fo:Fc: 0.946 / Cor.coef. Fo:Fc free: 0.919 / SU B: 2.341 / SU ML: 0.072 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.109 / ESU R Free: 0.108 Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 63.7 Å2 / Biso mean: 22.551 Å2 / Biso min: 9.99 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.8→18.78 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.8→1.846 Å / Rfactor Rfree error: 0 / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller










 PDBj
PDBj







