+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4h0h | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of mimicry-recognizing 2D10 scFv with peptide | ||||||
 Components Components |
| ||||||
 Keywords Keywords | IMMUNE SYSTEM / molecular mimicry / sugar | ||||||
| Function / homology | Immunoglobulins / Immunoglobulin-like / Sandwich / Mainly Beta Function and homology information Function and homology information | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2 Å MOLECULAR REPLACEMENT / Resolution: 2 Å | ||||||
 Authors Authors | Tapryal, S. / Gaur, V. / Kaur, K.J. / Salunke, D.M. | ||||||
 Citation Citation |  Journal: J.Immunol. / Year: 2013 Journal: J.Immunol. / Year: 2013Title: Structural evaluation of a mimicry-recognizing paratope: plasticity in antigen-antibody interactions manifests in molecular mimicry. Authors: Tapryal, S. / Gaur, V. / Kaur, K.J. / Salunke, D.M. | ||||||
| History |
|
- Structure visualization
Structure visualization
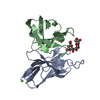
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4h0h.cif.gz 4h0h.cif.gz | 65.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4h0h.ent.gz pdb4h0h.ent.gz | 48.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4h0h.json.gz 4h0h.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/h0/4h0h https://data.pdbj.org/pub/pdb/validation_reports/h0/4h0h ftp://data.pdbj.org/pub/pdb/validation_reports/h0/4h0h ftp://data.pdbj.org/pub/pdb/validation_reports/h0/4h0h | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4h0gC  4h0iSC C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Antibody | Mass: 26894.973 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   |
|---|---|
| #2: Protein/peptide | Mass: 1365.444 Da / Num. of mol.: 1 / Source method: obtained synthetically |
| #3: Water | ChemComp-HOH / |
| Has protein modification | Y |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.47 Å3/Da / Density % sol: 50.23 % |
|---|---|
| Crystal grow | Temperature: 277 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: 100 mM MES, pH 6.5, 1.6 M magnesium sulfate, VAPOR DIFFUSION, HANGING DROP, temperature 277K |
-Data collection
| Diffraction | Mean temperature: 120 K |
|---|---|
| Diffraction source | Source:  ROTATING ANODE / Type: RIGAKU RU300 / Wavelength: 1.5418 Å ROTATING ANODE / Type: RIGAKU RU300 / Wavelength: 1.5418 Å |
| Detector | Type: MAR scanner 345 mm plate / Detector: IMAGE PLATE / Date: Dec 16, 2008 / Details: mirrors |
| Radiation | Monochromator: Ni filter CMF 12 38CU-6 (Osmic Inc.) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.5418 Å / Relative weight: 1 |
| Reflection | Resolution: 2→69.568 Å / Num. all: 19310 / Num. obs: 19310 / % possible obs: 100 % / Redundancy: 10.5 % / Rmerge(I) obs: 0.065 / Net I/σ(I): 24.1 |
| Reflection shell | Resolution: 2→2.11 Å / Redundancy: 10.1 % / Rmerge(I) obs: 0.427 / Mean I/σ(I) obs: 5.5 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 4H0I Resolution: 2→25.5 Å / Cor.coef. Fo:Fc: 0.95 / Cor.coef. Fo:Fc free: 0.942 / SU B: 3.385 / SU ML: 0.096 / Cross valid method: THROUGHOUT / ESU R: 0.197 / ESU R Free: 0.156 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: THE COORDINATES FOR RESIDUES 4-9 OF CHAIN D HAVE BEEN MODELED WHILE VISUALIZING THE 2FO-FC AND FO-FC MAPS AT SIGMA CUT-OFFS OF 0.7 AND 1.6, RESPECTIVELY, WITH AN OCCUPANCY OF 0.8 AND AN ...Details: THE COORDINATES FOR RESIDUES 4-9 OF CHAIN D HAVE BEEN MODELED WHILE VISUALIZING THE 2FO-FC AND FO-FC MAPS AT SIGMA CUT-OFFS OF 0.7 AND 1.6, RESPECTIVELY, WITH AN OCCUPANCY OF 0.8 AND AN AVERAGE B-FACTOR OF 92.1. THEREFORE, HIGHER REAL SPACE R-FACTORS AND LOWER DENSITY CORRELATION ARE EXPECTED FOR THESE RESIDUES.
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.4 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 30.55 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2→25.5 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2→2.052 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller












 PDBj
PDBj


