[English] 日本語
 Yorodumi
Yorodumi- PDB-4dwv: Horse alcohol dehydrogenase complexed with NAD+ and 2,3,4,5,6-pen... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4dwv | ||||||
|---|---|---|---|---|---|---|---|
| Title | Horse alcohol dehydrogenase complexed with NAD+ and 2,3,4,5,6-pentafluorobenzyl alcohol | ||||||
 Components Components | Alcohol dehydrogenase E chain | ||||||
 Keywords Keywords | OXIDOREDUCTASE / ALCOHOL DEHYDROGENASE / NAD+ / PENTAFLUOROBENZYL ALCOHOL / MICHAELIS COMPLEX / ROSSMANN FOLD | ||||||
| Function / homology |  Function and homology information Function and homology informationall-trans-retinol dehydrogenase (NAD+) activity / alcohol dehydrogenase / retinoic acid metabolic process / retinol metabolic process / zinc ion binding / cytosol Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.14 Å MOLECULAR REPLACEMENT / Resolution: 1.14 Å | ||||||
 Authors Authors | Plapp, B.V. / Ramaswamy, S. | ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2012 Journal: Biochemistry / Year: 2012Title: Atomic-Resolution Structures of Horse Liver Alcohol Dehydrogenase with NAD(+) and Fluoroalcohols Define Strained Michaelis Complexes. Authors: Plapp, B.V. / Ramaswamy, S. #1:  Journal: Biochemistry / Year: 1994 Journal: Biochemistry / Year: 1994Title: Structures of horse liver alcohol dehydrogenase complexed with NAD+ and substituted benzyl alcohols. Authors: Ramaswamy, S. / Eklund, H. / Plapp, B.V. #2:  Journal: Biochemistry / Year: 2003 Journal: Biochemistry / Year: 2003Title: Amino acid residues in the nicotinamide binding site contribute to catalysis by horse liver alcohol dehydrogenase. Authors: Rubach, J.K. / Plapp, B.V. #3:  Journal: J.Biol.Chem. / Year: 2003 Journal: J.Biol.Chem. / Year: 2003Title: Formamides mimic aldehydes and inhibit liver alcohol dehydrogenases and ethanol metabolism. Authors: Venkataramaiah, T.H. / Plapp, B.V. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4dwv.cif.gz 4dwv.cif.gz | 348.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4dwv.ent.gz pdb4dwv.ent.gz | 282.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4dwv.json.gz 4dwv.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/dw/4dwv https://data.pdbj.org/pub/pdb/validation_reports/dw/4dwv ftp://data.pdbj.org/pub/pdb/validation_reports/dw/4dwv ftp://data.pdbj.org/pub/pdb/validation_reports/dw/4dwv | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  4dxhC  1hldS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | |
| Experimental dataset #1 | Data reference:  10.15785/SBGRID/675 / Data set type: diffraction image data 10.15785/SBGRID/675 / Data set type: diffraction image data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 39853.273 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)  |
|---|
-Non-polymers , 5 types, 1052 molecules 
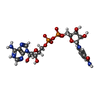
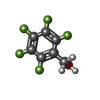






| #2: Chemical | ChemComp-ZN / #3: Chemical | #4: Chemical | #5: Chemical | ChemComp-MRD / ( #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.4 Å3/Da / Density % sol: 48.77 % |
|---|---|
| Crystal grow | Temperature: 278 K / Method: microdialysis / pH: 6.7 Details: 50 mm ammonium n-[tris(hydroxymethyl) methyl]-2-aminoethane sulfonate, ph 6.7 (at 25 c), 0.25 mm edta, 10 mg/ml protein, 1 mm nad+, 10 mm 2,3,4,5,6-pentafluorobenzyl alcohol, 12 to 25 % 2- ...Details: 50 mm ammonium n-[tris(hydroxymethyl) methyl]-2-aminoethane sulfonate, ph 6.7 (at 25 c), 0.25 mm edta, 10 mg/ml protein, 1 mm nad+, 10 mm 2,3,4,5,6-pentafluorobenzyl alcohol, 12 to 25 % 2-methyl-2,4-pentanediol, MICRODIALYSIS, temperature 278k |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 23-ID-D / Wavelength: 0.9537 Å / Beamline: 23-ID-D / Wavelength: 0.9537 Å |
| Detector | Type: MARMOSAIC 300 mm CCD / Detector: CCD / Date: Mar 9, 2006 / Details: ADJUSTABLE FOCUS K-B PAIR SI PLUS PT, RH COATINGS |
| Radiation | Monochromator: DOUBLE CRYSTAL CRYOCOOLED Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9537 Å / Relative weight: 1 |
| Reflection | Resolution: 1.14→20 Å / Num. all: 270489 / Num. obs: 253394 / % possible obs: 93.7 % / Observed criterion σ(F): 1 / Observed criterion σ(I): 1 / Redundancy: 4.75 % / Rmerge(I) obs: 0.065 / Net I/σ(I): 12.9 |
| Reflection shell | Resolution: 1.14→1.18 Å / Redundancy: 3.74 % / Rmerge(I) obs: 0.264 / Mean I/σ(I) obs: 3.2 / % possible all: 84.5 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: pdb entry 1HLD Resolution: 1.14→20 Å / Cor.coef. Fo:Fc: 0.985 / Cor.coef. Fo:Fc free: 0.983 / SU B: 0.961 / SU ML: 0.02 / Cross valid method: THROUGHOUT / ESU R: 0.028 / ESU R Free: 0.028 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 14.926 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.14→20 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.14→1.17 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller

























 PDBj
PDBj





