[English] 日本語
 Yorodumi
Yorodumi- PDB-4bul: Novel hydroxyl tricyclics (e.g. GSK966587) as potent inhibitors o... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4bul | ||||||
|---|---|---|---|---|---|---|---|
| Title | Novel hydroxyl tricyclics (e.g. GSK966587) as potent inhibitors of bacterial type IIA topoisomerases | ||||||
 Components Components |
| ||||||
 Keywords Keywords | ISOMERASE / TYPE IIA TOPOISOMERASES / NBTIS | ||||||
| Function / homology |  Function and homology information Function and homology informationDNA topoisomerase type II (double strand cut, ATP-hydrolyzing) complex / DNA negative supercoiling activity / DNA topoisomerase (ATP-hydrolysing) / DNA topological change / DNA-templated DNA replication / chromosome / response to antibiotic / DNA binding / ATP binding / metal ion binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  SYNTHETIC CONSTRUCT (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.6 Å MOLECULAR REPLACEMENT / Resolution: 2.6 Å | ||||||
 Authors Authors | Miles, T.J. / Hennessy, A.J. / Bax, B. / Brooks, G. / Brown, B.S. / Brown, P. / Cailleau, N. / Chen, D. / Dabbs, S. / Davies, D.T. ...Miles, T.J. / Hennessy, A.J. / Bax, B. / Brooks, G. / Brown, B.S. / Brown, P. / Cailleau, N. / Chen, D. / Dabbs, S. / Davies, D.T. / Esken, J.M. / Giordano, I. / Hoover, J.L. / Huang, J. / Jones, G.E. / Sukmar, S.K.K. / Spitzfaden, C. / Markwell, R.E. / Minthorn, E.A. / Rittenhouse, S. / Gwynn, M.N. / Pearson, N.D. | ||||||
 Citation Citation |  Journal: Bioorg.Med.Chem.Lett. / Year: 2013 Journal: Bioorg.Med.Chem.Lett. / Year: 2013Title: Novel Hydroxyl Tricyclics (E.G., Gsk966587) as Potent Inhibitors of Bacterial Type Iia Topoisomerases. Authors: Miles, T.J. / Hennessy, A.J. / Bax, B. / Brooks, G. / Brown, B.S. / Brown, P. / Cailleau, N. / Chen, D. / Dabbs, S. / Davies, D.T. / Esken, J.M. / Giordano, I. / Hoover, J.L. / Huang, J. / ...Authors: Miles, T.J. / Hennessy, A.J. / Bax, B. / Brooks, G. / Brown, B.S. / Brown, P. / Cailleau, N. / Chen, D. / Dabbs, S. / Davies, D.T. / Esken, J.M. / Giordano, I. / Hoover, J.L. / Huang, J. / Jones, G.E. / Kusalakumari Sukmar, S.K. / Spitzfaden, C. / Markwell, R.E. / Minthorn, E.A. / Rittenhouse, S. / Gwynn, M.N. / Pearson, N.D. #1:  Journal: Nature / Year: 2010 Journal: Nature / Year: 2010Title: Type Iia Topoisomerase Inhibition by a New Class of Antibacterial Agents. Authors: Bax, B.D. / Chan, P.F. / Eggleston, D.S. / Fosberry, A. / Gentry, D.R. / Gorrec, F. / Giordano, I. / Hann, M.M. / Hennessy, A. / Hibbs, M. / Huang, J. / Jones, E. / Jones, J. / Brown, K.K. / ...Authors: Bax, B.D. / Chan, P.F. / Eggleston, D.S. / Fosberry, A. / Gentry, D.R. / Gorrec, F. / Giordano, I. / Hann, M.M. / Hennessy, A. / Hibbs, M. / Huang, J. / Jones, E. / Jones, J. / Brown, K.K. / Lewis, C.J. / May, E.W. / Saunders, M.R. / Singh, O. / Spitzfaden, C.E. / Shen, C. / Shillings, A. / Theobald, A.J. / Wohlkonig, A. / Pearson, N.D. / Gwynn, M.N. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4bul.cif.gz 4bul.cif.gz | 627.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4bul.ent.gz pdb4bul.ent.gz | 511.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4bul.json.gz 4bul.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/bu/4bul https://data.pdbj.org/pub/pdb/validation_reports/bu/4bul ftp://data.pdbj.org/pub/pdb/validation_reports/bu/4bul ftp://data.pdbj.org/pub/pdb/validation_reports/bu/4bul | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2xcsS S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 2 molecules AC
| #1: Protein | Mass: 78109.000 Da / Num. of mol.: 2 Fragment: GYRA N-TERMINAL 56KDA DOMAIN, RESIDUES 2-491, GYRB C-TERMINAL 27KDA DOMAIN, RESIDUES 410-644 Mutation: YES Source method: isolated from a genetically manipulated source Details: FUSION TRUNCATE WITH DELETION AND Y123F GYRB27A56GKDELY123F (BAX ET AL., 2010) Source: (gene. exp.)   References: UniProt: P66937, UniProt: Q99XG5, EC: 5.99.1.3, EC: 5.99.1.3 |
|---|
-DNA chain , 2 types, 4 molecules EGFH
| #2: DNA chain | Mass: 6156.963 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.) SYNTHETIC CONSTRUCT (others) #3: DNA chain | Mass: 6112.970 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.) SYNTHETIC CONSTRUCT (others) |
|---|
-Non-polymers , 3 types, 453 molecules 
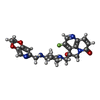



| #4: Chemical | ChemComp-MN / #5: Chemical | ChemComp-AE8 / ( | #6: Water | ChemComp-HOH / | |
|---|
-Details
| Nonpolymer details | GSK966587 (S)-4-((4-(((2,3-DIHYDRO-[1,4]DIOXINO[2, 3-C]PYRIDIN-7-YL)METHYL)AMINO)PIPERIDIN-1-YL) ...GSK966587 (S)-4-((4-(((2,3-DIHYDRO-[1,4]DIOXINO[2, 3-C]PYRIDIN-7-YL)METHYL)AMINO)PIPERIDIN-1-YL)METHYL)- 3-FLUORO-4-HYDROXY-4H-PYRROLO[3,2,1-DE][1,5] NAPHTHYRID |
|---|---|
| Sequence details | C-TERMINAL DOMAIN (410-644) WITH GREEK KEY DOMAIN (544-579) DELETED AND REPLACED WITH TWO RESIDUES, ...C-TERMINAL DOMAIN (410-644) WITH GREEK KEY DOMAIN (544-579) DELETED AND REPLACED WITH TWO RESIDUES, TG. CATALYTIC TYROSINE MUTATED TO PHENYLALAN |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.15 Å3/Da / Density % sol: 60.94 % / Description: NONE |
|---|
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-1 / Wavelength: 0.96865 / Beamline: ID23-1 / Wavelength: 0.96865 | |||||||||||||||
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD | |||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||
| Radiation wavelength | Wavelength: 0.96865 Å / Relative weight: 1 | |||||||||||||||
| Reflection twin |
| |||||||||||||||
| Reflection | Resolution: 2.6→81.38 Å / Num. obs: 60721 / % possible obs: 95.8 % / Observed criterion σ(I): 0 / Redundancy: 2.4 % / Rmerge(I) obs: 0.09 / Net I/σ(I): 9.5 | |||||||||||||||
| Reflection shell | Resolution: 2.6→2.74 Å / Redundancy: 2.4 % / Rmerge(I) obs: 0.38 / Mean I/σ(I) obs: 2.3 / % possible all: 96.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 2XCS Resolution: 2.6→24.79 Å / Cor.coef. Fo:Fc: 0.959 / Cor.coef. Fo:Fc free: 0.932 / SU B: 11.49 / SU ML: 0.136 / Cross valid method: THROUGHOUT / ESU R: 0.107 / ESU R Free: 0.048 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: U VALUES WITH TLS ADDED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: BABINET MODEL WITH MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.6→24.79 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj







































