+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4avp | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the DNA-binding domain of human ETV1. | ||||||
 Components Components | ETS TRANSLOCATION VARIANT 1 | ||||||
 Keywords Keywords | TRANSCRIPTION / TRANSCRIPTIONAL ACTIVATION AND REPRESSION / DNA BINDING PROTEIN / E TWENTY-SIX / ERWING SARCOMA / PROSTATE CANCER / MELANOMA / GASTROINTESTINAL STROMAL TUMOUR | ||||||
| Function / homology |  Function and homology information Function and homology informationperipheral nervous system neuron development / mechanosensory behavior / muscle organ development / axon guidance / sequence-specific double-stranded DNA binding / DNA-binding transcription activator activity, RNA polymerase II-specific / transcription by RNA polymerase II / DNA-binding transcription factor activity, RNA polymerase II-specific / cell differentiation / RNA polymerase II cis-regulatory region sequence-specific DNA binding ...peripheral nervous system neuron development / mechanosensory behavior / muscle organ development / axon guidance / sequence-specific double-stranded DNA binding / DNA-binding transcription activator activity, RNA polymerase II-specific / transcription by RNA polymerase II / DNA-binding transcription factor activity, RNA polymerase II-specific / cell differentiation / RNA polymerase II cis-regulatory region sequence-specific DNA binding / DNA-binding transcription factor activity / regulation of transcription by RNA polymerase II / chromatin / positive regulation of transcription by RNA polymerase II / nucleus Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.82 Å MOLECULAR REPLACEMENT / Resolution: 1.82 Å | ||||||
 Authors Authors | Allerston, C.K. / Cooper, C.D.O. / Krojer, T. / Chaikuad, A. / Filippakopoulos, P. / Canning, P. / Arrowsmith, C.H. / Edwards, A. / Bountra, C. / von Delft, F. / Gileadi, O. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2015 Journal: J.Biol.Chem. / Year: 2015Title: Structures of the Ets Domains of Transcription Factors Etv1, Etv4, Etv5 and Fev: Determinants of DNA Binding and Redox Regulation by Disulfide Bond Formation. Authors: Cooper, C.D.O. / Newman, J.A. / Aitkenhead, H. / Allerston, C.K. / Gileadi, O. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4avp.cif.gz 4avp.cif.gz | 92.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4avp.ent.gz pdb4avp.ent.gz | 69.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4avp.json.gz 4avp.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  4avp_validation.pdf.gz 4avp_validation.pdf.gz | 463.9 KB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  4avp_full_validation.pdf.gz 4avp_full_validation.pdf.gz | 465.4 KB | Display | |
| Data in XML |  4avp_validation.xml.gz 4avp_validation.xml.gz | 17.7 KB | Display | |
| Data in CIF |  4avp_validation.cif.gz 4avp_validation.cif.gz | 24.6 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/av/4avp https://data.pdbj.org/pub/pdb/validation_reports/av/4avp ftp://data.pdbj.org/pub/pdb/validation_reports/av/4avp ftp://data.pdbj.org/pub/pdb/validation_reports/av/4avp | HTTPS FTP |
-Related structure data
| Related structure data |  2yprC  3zp5C 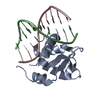 4bncC  4co8C  4unoC  4uuvC  1gvjS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
| ||||||||
| Noncrystallographic symmetry (NCS) | NCS oper: (Code: given) |
- Components
Components
| #1: Protein | Mass: 12437.209 Da / Num. of mol.: 4 / Fragment: DNA-BINDING DOMAIN, RESIDUES 326-429 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Plasmid: PNIC28-BSA4 / Production host: HOMO SAPIENS (human) / Plasmid: PNIC28-BSA4 / Production host:  #2: Chemical | #3: Water | ChemComp-HOH / | Compound details | ENGINEERED RESIDUE IN CHAIN A, TYR 329 TO SER ENGINEERED RESIDUE IN CHAIN B, TYR 329 TO SER ...ENGINEERED | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.72 Å3/Da / Density % sol: 28.55 % / Description: NONE |
|---|---|
| Crystal grow | pH: 7.5 / Details: 2.5M SODIUM FORMATE, pH 7.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 0.9795 / Beamline: I04 / Wavelength: 0.9795 |
| Detector | Type: MARRESEARCH MARMOSAIC 300 / Detector: CCD / Date: Apr 29, 2012 / Details: MIRRORS |
| Radiation | Monochromator: DOUBLE CRYSTAL / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9795 Å / Relative weight: 1 |
| Reflection | Resolution: 1.82→53.94 Å / Num. obs: 27819 / % possible obs: 97.4 % / Observed criterion σ(I): 2 / Redundancy: 2.3 % / Biso Wilson estimate: 22.04 Å2 / Rmerge(I) obs: 0.054 / Rpim(I) all: 0.062 / Net I/σ(I): 10.4 |
| Reflection shell | Resolution: 1.82→1.92 Å / Redundancy: 2.3 % / Rmerge(I) obs: 0.33 / Mean I/σ(I) obs: 2.3 / Rpim(I) all: 0.36 / % possible all: 96.2 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1GVJ Resolution: 1.82→53.94 Å / Cor.coef. Fo:Fc: 0.9259 / Cor.coef. Fo:Fc free: 0.8979 / SU R Cruickshank DPI: 0.19 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.187 / SU Rfree Blow DPI: 0.159 / SU Rfree Cruickshank DPI: 0.161 Details: IDEAL-DIST CONTACT TERM CONTACT SETUP. ALL ATOMS HAVE CCP4 ATOM TYPE FROM LIBRARY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 23.4 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.82→53.94 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.82→1.89 Å / Total num. of bins used: 14
|
 Movie
Movie Controller
Controller













 PDBj
PDBj


