[English] 日本語
 Yorodumi
Yorodumi- PDB-2ypr: Crystal structure of the DNA binding ETS domain of human protein FEV -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2ypr | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the DNA binding ETS domain of human protein FEV | ||||||
 Components Components | PROTEIN FEV | ||||||
 Keywords Keywords | TRANSCRIPTION | ||||||
| Function / homology |  Function and homology information Function and homology informationneuron fate specification / neuron maturation / maternal behavior / sequence-specific double-stranded DNA binding / DNA-binding transcription activator activity, RNA polymerase II-specific / DNA-binding transcription factor activity, RNA polymerase II-specific / cell differentiation / nuclear speck / RNA polymerase II cis-regulatory region sequence-specific DNA binding / positive regulation of gene expression ...neuron fate specification / neuron maturation / maternal behavior / sequence-specific double-stranded DNA binding / DNA-binding transcription activator activity, RNA polymerase II-specific / DNA-binding transcription factor activity, RNA polymerase II-specific / cell differentiation / nuclear speck / RNA polymerase II cis-regulatory region sequence-specific DNA binding / positive regulation of gene expression / regulation of transcription by RNA polymerase II / chromatin / nucleus Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.64 Å MOLECULAR REPLACEMENT / Resolution: 2.64 Å | ||||||
 Authors Authors | Allerston, C.K. / Cooper, C. / Vollmar, M. / Krojer, T. / von Delft, F. / Weigelt, J. / Arrowsmith, C.H. / Bountra, C. / Edwards, A. / Gileadi, O. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2015 Journal: J.Biol.Chem. / Year: 2015Title: Structures of the Ets Domains of Transcription Factors Etv1, Etv4, Etv5 and Fev: Determinants of DNA Binding and Redox Regulation by Disulfide Bond Formation. Authors: Cooper, C.D.O. / Newman, J.A. / Aitkenhead, H. / Allerston, C.K. / Gileadi, O. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2ypr.cif.gz 2ypr.cif.gz | 88.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2ypr.ent.gz pdb2ypr.ent.gz | 68.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2ypr.json.gz 2ypr.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/yp/2ypr https://data.pdbj.org/pub/pdb/validation_reports/yp/2ypr ftp://data.pdbj.org/pub/pdb/validation_reports/yp/2ypr ftp://data.pdbj.org/pub/pdb/validation_reports/yp/2ypr | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3zp5C  4avpC 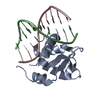 4bncC  4co8C  4unoC  4uuvC  1fliS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 11813.431 Da / Num. of mol.: 2 / Fragment: ETS DOMAIN, RESIDUES 42-141 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Plasmid: PNIC28-BSA4 / Production host: HOMO SAPIENS (human) / Plasmid: PNIC28-BSA4 / Production host:  #2: Chemical | ChemComp-GOL / | #3: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.96 Å3/Da / Density % sol: 68.95 % / Description: NONE |
|---|---|
| Crystal grow | Details: 14% PEG3350, 5% GLYCEROL, 100 MM CITRATE PH 5.3 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I02 / Wavelength: 0.9795 / Beamline: I02 / Wavelength: 0.9795 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: Sep 26, 2012 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9795 Å / Relative weight: 1 |
| Reflection | Resolution: 2.64→75.5 Å / Num. obs: 11808 / % possible obs: 100 % / Observed criterion σ(I): 2 / Redundancy: 15.9 % / Biso Wilson estimate: 102.82 Å2 / Rmerge(I) obs: 0.09 / Net I/σ(I): 19.4 |
| Reflection shell | Resolution: 2.64→2.77 Å / Redundancy: 16.8 % / Mean I/σ(I) obs: 2 / % possible all: 100 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1FLI Resolution: 2.64→75.5 Å / Cor.coef. Fo:Fc: 0.9357 / Cor.coef. Fo:Fc free: 0.9495 / SU R Cruickshank DPI: 0.289 / Cross valid method: THROUGHOUT / σ(F): 0 / SU R Blow DPI: 0.296 / SU Rfree Blow DPI: 0.214 / SU Rfree Cruickshank DPI: 0.213
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 87.06 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine analyze | Luzzati coordinate error obs: 0.449 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.64→75.5 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.64→2.89 Å / Total num. of bins used: 6
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj


