[English] 日本語
 Yorodumi
Yorodumi- PDB-4uno: Crystal structure of the ETS domain of human ETV5 in complex with DNA -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4uno | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the ETS domain of human ETV5 in complex with DNA | ||||||
 Components Components |
| ||||||
 Keywords Keywords | DNA BINDING PROTEIN | ||||||
| Function / homology |  Function and homology information Function and homology informationmale germ-line stem cell asymmetric division / regulation of branching involved in mammary gland duct morphogenesis / skeletal muscle acetylcholine-gated channel clustering / neuromuscular synaptic transmission / regulation of synapse organization / positive regulation of glial cell proliferation / positive regulation of neuron differentiation / RNA polymerase II transcription regulatory region sequence-specific DNA binding / locomotory behavior / sequence-specific double-stranded DNA binding ...male germ-line stem cell asymmetric division / regulation of branching involved in mammary gland duct morphogenesis / skeletal muscle acetylcholine-gated channel clustering / neuromuscular synaptic transmission / regulation of synapse organization / positive regulation of glial cell proliferation / positive regulation of neuron differentiation / RNA polymerase II transcription regulatory region sequence-specific DNA binding / locomotory behavior / sequence-specific double-stranded DNA binding / DNA-binding transcription activator activity, RNA polymerase II-specific / cellular response to oxidative stress / DNA-binding transcription factor activity, RNA polymerase II-specific / cell differentiation / transcription cis-regulatory region binding / DNA-binding transcription factor activity / synapse / regulation of transcription by RNA polymerase II / chromatin / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / DNA binding / nucleoplasm / nucleus / plasma membrane Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human)SYNTHETIC CONSTRUCT (others) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.95 Å MOLECULAR REPLACEMENT / Resolution: 1.95 Å | ||||||
 Authors Authors | Newman, J.A. / Aitkenhead, H. / Cooper, C.D.O. / Pinkas, D.M. / Shrestha, L. / Burgess-Brown, N. / Kopec, J. / Fitzpatrick, F. / Tallant, C. / von Delft, F. ...Newman, J.A. / Aitkenhead, H. / Cooper, C.D.O. / Pinkas, D.M. / Shrestha, L. / Burgess-Brown, N. / Kopec, J. / Fitzpatrick, F. / Tallant, C. / von Delft, F. / Arrowsmith, C.H. / Bountra, C. / Edwards, A. / Gileadi, O. | ||||||
 Citation Citation |  Journal: J.Biol.Chem. / Year: 2015 Journal: J.Biol.Chem. / Year: 2015Title: Structures of the Ets Domains of Transcription Factors Etv1, Etv4, Etv5 and Fev: Determinants of DNA Binding and Redox Regulation by Disulfide Bond Formation. Authors: Cooper, C.D.O. / Newman, J.A. / Aitkenhead, H. / Allerston, C.K. / Gileadi, O. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4uno.cif.gz 4uno.cif.gz | 49.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4uno.ent.gz pdb4uno.ent.gz | 31.9 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  4uno.json.gz 4uno.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/un/4uno https://data.pdbj.org/pub/pdb/validation_reports/un/4uno ftp://data.pdbj.org/pub/pdb/validation_reports/un/4uno ftp://data.pdbj.org/pub/pdb/validation_reports/un/4uno | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2yprC  3zp5C  4avpC 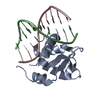 4bncSC  4co8C  4uuvC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 11803.574 Da / Num. of mol.: 1 / Fragment: ETS DOMAIN, RESIDUES 365-462 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Production host: HOMO SAPIENS (human) / Production host:  | ||||||
|---|---|---|---|---|---|---|---|
| #2: DNA chain | Mass: 3094.042 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) SYNTHETIC CONSTRUCT (others) | ||||||
| #3: DNA chain | Mass: 2995.967 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.) SYNTHETIC CONSTRUCT (others) | ||||||
| #4: Chemical | | #5: Water | ChemComp-HOH / | Has protein modification | Y | Sequence details | FIRST 2 RESIDUES REMAIN AFTER TAG CLEAVAGE | |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.3 Å3/Da / Density % sol: 46.53 % / Description: NONE |
|---|---|
| Crystal grow | Details: 40% PEG 300, 0.2M CALCIUM ACETATE, 0.1M CACODYLATE PH 6.0 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I03 / Wavelength: 0.97625 / Beamline: I03 / Wavelength: 0.97625 |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Apr 24, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97625 Å / Relative weight: 1 |
| Reflection | Resolution: 1.95→19.52 Å / Num. obs: 12146 / % possible obs: 99.9 % / Observed criterion σ(I): -3 / Redundancy: 9.7 % / Biso Wilson estimate: 30.5 Å2 / Rmerge(I) obs: 0.08 / Net I/σ(I): 16.4 |
| Reflection shell | Resolution: 1.95→2 Å / Redundancy: 9 % / Rmerge(I) obs: 0.9 / Mean I/σ(I) obs: 2.3 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 4BNC Resolution: 1.95→19.515 Å / SU ML: 0.15 / σ(F): 1.35 / Phase error: 22.5 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 34.8 Å2 | |||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.95→19.515 Å
| |||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj


