+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3h9q | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of E. coli MccB + SeMet MccA | ||||||
 Components Components |
| ||||||
 Keywords Keywords | TRANSFERASE/ANTIBIOTIC / Ubiquitin-activating enzyme / microcin / bacteriocin / Mcc7 / peptide antibiotic / N-P bond formation / Antibiotic / Antimicrobial / Formylation / Phosphoprotein / TRANSFERASE / TRANSFERASE-ANTIBIOTIC COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology informationubiquitin-like modifier activating enzyme activity / thiosulfate-cyanide sulfurtransferase activity / nucleotidyltransferase activity / defense response to bacterium / ATP binding / metal ion binding / cytoplasm Similarity search - Function | ||||||
| Biological species |  | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  FOURIER SYNTHESIS / Resolution: 2.63 Å FOURIER SYNTHESIS / Resolution: 2.63 Å | ||||||
 Authors Authors | Regni, C.A. / Roush, R.F. / Miller, D. / Nourse, A. / Walsh, C.T. / Schulman, B.A. | ||||||
 Citation Citation |  Journal: Embo J. / Year: 2009 Journal: Embo J. / Year: 2009Title: How the MccB bacterial ancestor of ubiquitin E1 initiates biosynthesis of the microcin C7 antibiotic. Authors: Regni, C.A. / Roush, R.F. / Miller, D.J. / Nourse, A. / Walsh, C.T. / Schulman, B.A. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3h9q.cif.gz 3h9q.cif.gz | 270.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3h9q.ent.gz pdb3h9q.ent.gz | 216.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3h9q.json.gz 3h9q.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/h9/3h9q https://data.pdbj.org/pub/pdb/validation_reports/h9/3h9q ftp://data.pdbj.org/pub/pdb/validation_reports/h9/3h9q ftp://data.pdbj.org/pub/pdb/validation_reports/h9/3h9q | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  3h5aC  3h5nC  3h5rC  3h9gC  3h9jSC C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| 2 | 
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 39409.766 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Details: thrombin cleavable His-tag / Source: (gene. exp.)   #2: Protein/peptide | 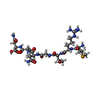  Type: Polypeptide / Class: Inhibitor / Mass: 810.739 Da / Num. of mol.: 4 / Source method: obtained synthetically Type: Polypeptide / Class: Inhibitor / Mass: 810.739 Da / Num. of mol.: 4 / Source method: obtained syntheticallyDetails: MccA peptide was synthesized and reverse-phase HPLC purified by the Hartwell Center for Bioinformatics and Biotechnology at St. Jude Children's Research Hospital Source: (synth.)  References: UniProt: Q47505, Selenomethionine derivative of Microcin C7 #3: Chemical | ChemComp-ZN / #4: Chemical | #5: Water | ChemComp-HOH / | Has protein modification | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.97 Å3/Da / Density % sol: 37.63 % |
|---|---|
| Crystal grow | Temperature: 291.2 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: 24-26% Pentaerythritol ethoxylate (15/4 EO/OH, Hampton Research), 50 mM Bis-Tris pH 6.5, 50 mM (NH4)2SO4, 10 mM SeMet-MccA, VAPOR DIFFUSION, HANGING DROP, temperature 291.2K |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  APS APS  / Beamline: 24-ID-E / Wavelength: 0.97926 Å / Beamline: 24-ID-E / Wavelength: 0.97926 Å |
| Detector | Type: ADSC QUANTUM 315 / Detector: CCD / Date: Nov 19, 2007 Details: Vertical and horizontal focusing mirrors in Kirkpatrick-Baez geometry. |
| Radiation | Monochromator: Cryogenically-cooled single crystal Si(111) side bounce, optional Si(311) Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.97926 Å / Relative weight: 1 |
| Reflection | Resolution: 2.63→20 Å / Num. all: 37055 / Num. obs: 37055 / % possible obs: 100 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 3.5 % / Biso Wilson estimate: 58.9 Å2 / Rmerge(I) obs: 0.083 / Χ2: 1.082 / Net I/σ(I): 23.312 |
| Reflection shell | Resolution: 2.63→2.72 Å / Redundancy: 3.5 % / Rmerge(I) obs: 0.311 / Num. unique all: 3683 / Χ2: 0.546 / % possible all: 99.9 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  FOURIER SYNTHESIS FOURIER SYNTHESISStarting model: PDB entry 3H9J Resolution: 2.63→20 Å / Occupancy max: 1 / Occupancy min: 0 / Isotropic thermal model: isotropic / Cross valid method: THROUGHOUT / σ(F): 0 / Stereochemistry target values: MAXIMUM LIKELIHOOD
| ||||||||||||||||||||||||||||||||
| Solvent computation | Bsol: 27.884 Å2 | ||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 123.34 Å2 / Biso mean: 51.983 Å2 / Biso min: 19.97 Å2
| ||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.63→20 Å
| ||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||
| Xplor file |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj






