[English] 日本語
 Yorodumi
Yorodumi- PDB-2w0x: FACTOR INHIBITING HIF-1 ALPHA WITH PYRIDINE 2,4 DICARBOXYLIC ACID -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 2w0x | ||||||
|---|---|---|---|---|---|---|---|
| Title | FACTOR INHIBITING HIF-1 ALPHA WITH PYRIDINE 2,4 DICARBOXYLIC ACID | ||||||
 Components Components | HYPOXIA-INDUCIBLE FACTOR 1 ALPHA INHIBITOR | ||||||
 Keywords Keywords | OXIDOREDUCTASE / HYDROXYLASE / DIOXYGENASE / TRANSCRIPTION / TRANSCRIPTION ACTIVATOR/INHIBITOR / HYPOXIA | ||||||
| Function / homology |  Function and homology information Function and homology informationhypoxia-inducible factor-asparagine dioxygenase / : / [protein]-asparagine 3-dioxygenase activity / peptidyl-histidine dioxygenase activity / peptidyl-aspartic acid 3-dioxygenase activity / regulation of vascular endothelial growth factor receptor signaling pathway / Cellular response to hypoxia / positive regulation of vasculogenesis / carboxylic acid binding / ankyrin repeat binding ...hypoxia-inducible factor-asparagine dioxygenase / : / [protein]-asparagine 3-dioxygenase activity / peptidyl-histidine dioxygenase activity / peptidyl-aspartic acid 3-dioxygenase activity / regulation of vascular endothelial growth factor receptor signaling pathway / Cellular response to hypoxia / positive regulation of vasculogenesis / carboxylic acid binding / ankyrin repeat binding / Notch binding / oxygen sensor activity / negative regulation of Notch signaling pathway / NF-kappaB binding / positive regulation of myoblast differentiation / ferrous iron binding / transcription corepressor activity / RNA polymerase II-specific DNA-binding transcription factor binding / perinuclear region of cytoplasm / negative regulation of transcription by RNA polymerase II / protein homodimerization activity / zinc ion binding / nucleoplasm / cytoplasm / cytosol Similarity search - Function | ||||||
| Biological species |  HOMO SAPIENS (human) HOMO SAPIENS (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.12 Å MOLECULAR REPLACEMENT / Resolution: 2.12 Å | ||||||
 Authors Authors | Conejo-Garcia, A. / Lienard, B.M.R. / Clifton, I.J. / McDonough, M.A. / Schofield, C.J. | ||||||
 Citation Citation |  Journal: Bioorg. Med. Chem. Lett. / Year: 2010 Journal: Bioorg. Med. Chem. Lett. / Year: 2010Title: Structural basis for binding of cyclic 2-oxoglutarate analogues to factor-inhibiting hypoxia-inducible factor. Authors: Conejo-Garcia, A. / McDonough, M.A. / Loenarz, C. / McNeill, L.A. / Hewitson, K.S. / Ge, W. / Lienard, B.M. / Schofield, C.J. / Clifton, I.J. #1:  Journal: J.Biol.Chem. / Year: 2003 Journal: J.Biol.Chem. / Year: 2003Title: Structure of Factor-Inhibiting Hypoxia-Inducible Factor (Hif) Reveals Mechanism of Oxidative Modification of Hif-1 Alpha. Authors: Elkins, J.M. / Hewitson, K.S. / McNeill, L.A. / Seibel, J.F. / Schlemminger, I. / Pugh, C.W. / Ratcliffe, P.J. / Schofield, C.J. | ||||||
| History |
| ||||||
| Remark 700 | SHEET THE SHEET STRUCTURE OF THIS MOLECULE IS BIFURCATED. IN ORDER TO REPRESENT THIS FEATURE IN ... SHEET THE SHEET STRUCTURE OF THIS MOLECULE IS BIFURCATED. IN ORDER TO REPRESENT THIS FEATURE IN THE SHEET RECORDS BELOW, TWO SHEETS ARE DEFINED. |
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  2w0x.cif.gz 2w0x.cif.gz | 90.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb2w0x.ent.gz pdb2w0x.ent.gz | 67.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  2w0x.json.gz 2w0x.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/w0/2w0x https://data.pdbj.org/pub/pdb/validation_reports/w0/2w0x ftp://data.pdbj.org/pub/pdb/validation_reports/w0/2w0x ftp://data.pdbj.org/pub/pdb/validation_reports/w0/2w0x | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  2wa3C  2wa4C  1h2nS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 40328.281 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  HOMO SAPIENS (human) / Plasmid: PET28A / Production host: HOMO SAPIENS (human) / Plasmid: PET28A / Production host:  References: UniProt: Q9NWT6, peptide-aspartate beta-dioxygenase |
|---|
-Non-polymers , 5 types, 206 molecules 
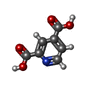







| #2: Chemical | ChemComp-FE2 / | ||||
|---|---|---|---|---|---|
| #3: Chemical | ChemComp-PD2 / | ||||
| #4: Chemical | | #5: Chemical | ChemComp-GOL / | #6: Water | ChemComp-HOH / | |
-Details
| Nonpolymer details | PYRIDINE-2,4-DICARBOXYL |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.43 Å3/Da / Density % sol: 64 % / Description: NONE |
|---|---|
| Crystal grow | pH: 7.5 Details: 1.6M AMMONIUM SULFATE, 0.1M HEPES PH 7.5, 5% PEG400, 1MM FESO4, 2MM SUBSTRATE |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SRS SRS  / Beamline: PX14.1 / Wavelength: 1.488 / Beamline: PX14.1 / Wavelength: 1.488 |
| Detector | Type: ADSC CCD / Detector: CCD / Date: May 10, 2005 / Details: RH COATED MIRROR |
| Radiation | Monochromator: SI (1 1 1) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 1.488 Å / Relative weight: 1 |
| Reflection | Resolution: 2.13→61.2 Å / Num. obs: 32255 / % possible obs: 99.9 % / Redundancy: 8.2 % / Biso Wilson estimate: 39.71 Å2 / Rmerge(I) obs: 0.08 / Net I/σ(I): 16.9 |
| Reflection shell | Resolution: 2.12→2.24 Å / Redundancy: 4.7 % / Rmerge(I) obs: 0.57 / Mean I/σ(I) obs: 1.8 / % possible all: 99.7 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: PDB ENTRY 1H2N Resolution: 2.12→38.67 Å / SU ML: 0.34 / σ(F): 0.03 / Phase error: 27.54 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL / Bsol: 52.63 Å2 / ksol: 0.36 e/Å3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 50.43 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.12→38.67 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
|
 Movie
Movie Controller
Controller































 PDBj
PDBj









