+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3703 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human cytoplasmic dynein-1 tail in the twisted state | |||||||||
 Map data Map data | Tail region particles were recentered and extracted from the complete human dynein-1 complex. Particles predominantly contain the dynein heavy chain N-terminus in a twisted conformation. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Motor protein / dynein / tail complex | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
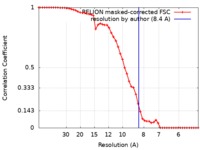
| Method | single particle reconstruction / cryo EM / Resolution: 8.4 Å | |||||||||
 Authors Authors | Zhang K | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2017 Journal: Cell / Year: 2017Title: Cryo-EM Reveals How Human Cytoplasmic Dynein Is Auto-inhibited and Activated. Authors: Kai Zhang / Helen E Foster / Arnaud Rondelet / Samuel E Lacey / Nadia Bahi-Buisson / Alexander W Bird / Andrew P Carter /    Abstract: Cytoplasmic dynein-1 binds dynactin and cargo adaptor proteins to form a transport machine capable of long-distance processive movement along microtubules. However, it is unclear why dynein-1 moves ...Cytoplasmic dynein-1 binds dynactin and cargo adaptor proteins to form a transport machine capable of long-distance processive movement along microtubules. However, it is unclear why dynein-1 moves poorly on its own or how it is activated by dynactin. Here, we present a cryoelectron microscopy structure of the complete 1.4-megadalton human dynein-1 complex in an inhibited state known as the phi-particle. We reveal the 3D structure of the cargo binding dynein tail and show how self-dimerization of the motor domains locks them in a conformation with low microtubule affinity. Disrupting motor dimerization with structure-based mutagenesis drives dynein-1 into an open form with higher affinity for both microtubules and dynactin. We find the open form is also inhibited for movement and that dynactin relieves this by reorienting the motor domains to interact correctly with microtubules. Our model explains how dynactin binding to the dynein-1 tail directly stimulates its motor activity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3703.map.gz emd_3703.map.gz | 649.8 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3703-v30.xml emd-3703-v30.xml emd-3703.xml emd-3703.xml | 38.6 KB 38.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3703_fsc.xml emd_3703_fsc.xml | 5 KB | Display |  FSC data file FSC data file |
| Images |  emd_3703.png emd_3703.png | 72.2 KB | ||
| Filedesc metadata |  emd-3703.cif.gz emd-3703.cif.gz | 5.1 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3703 http://ftp.pdbj.org/pub/emdb/structures/EMD-3703 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3703 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3703 | HTTPS FTP |
-Related structure data
| Related structure data |  5nvsMC  3698C  3704C  3705C  3706C  3707C  5nugC  5nvuC  5nw4C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_3703.map.gz / Format: CCP4 / Size: 11.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3703.map.gz / Format: CCP4 / Size: 11.4 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Tail region particles were recentered and extracted from the complete human dynein-1 complex. Particles predominantly contain the dynein heavy chain N-terminus in a twisted conformation. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.64 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
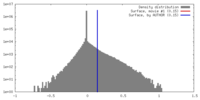
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
+Entire : Human cytoplasmic dynein-1 tail in the twisted N-terminus state
+Supramolecule #1: Human cytoplasmic dynein-1 tail in the twisted N-terminus state
+Macromolecule #1: dynein heavy chain
+Macromolecule #2: dynein intermediate chain
+Macromolecule #3: dynein heavy chain
+Macromolecule #4: dynein light intermediate chain
+Macromolecule #5: dynein light intermediate chain
+Macromolecule #6: N-terminal dimerization domain
+Macromolecule #7: N-terminal dimerization domain
+Macromolecule #8: LC8
+Macromolecule #9: Tctex
+Macromolecule #10: Tctex
+Macromolecule #11: intermediate chain
+Macromolecule #12: intermediate chain
+Macromolecule #13: Robl
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL |
|---|---|
| Buffer | pH: 7.4 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Average electron dose: 1.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)