[English] 日本語
 Yorodumi
Yorodumi- EMDB-3698: motor domain of complete human dynein-1 in the state of phi-particle -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-3698 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | motor domain of complete human dynein-1 in the state of phi-particle | |||||||||
 Map data Map data | locally masked region of motor domain from complete human dynein-1 phi-particle | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | motor protein / dynein / motor domain / AAA+ | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of intracellular transport / regulation of metaphase plate congression / positive regulation of spindle assembly / establishment of spindle localization / dynein complex / COPI-independent Golgi-to-ER retrograde traffic / retrograde axonal transport / P-body assembly / minus-end-directed microtubule motor activity / dynein light intermediate chain binding ...positive regulation of intracellular transport / regulation of metaphase plate congression / positive regulation of spindle assembly / establishment of spindle localization / dynein complex / COPI-independent Golgi-to-ER retrograde traffic / retrograde axonal transport / P-body assembly / minus-end-directed microtubule motor activity / dynein light intermediate chain binding / cytoplasmic dynein complex / nuclear migration / dynein intermediate chain binding / COPI-mediated anterograde transport / cytoplasmic microtubule / cytoplasmic microtubule organization / Amplification of signal from unattached kinetochores via a MAD2 inhibitory signal / axon cytoplasm / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / Recruitment of mitotic centrosome proteins and complexes / MHC class II antigen presentation / Mitotic Prometaphase / Recruitment of NuMA to mitotic centrosomes / EML4 and NUDC in mitotic spindle formation / Anchoring of the basal body to the plasma membrane / stress granule assembly / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / regulation of mitotic spindle organization / Resolution of Sister Chromatid Cohesion / AURKA Activation by TPX2 / mitotic spindle organization / filopodium / RHO GTPases Activate Formins / HCMV Early Events / Aggrephagy / azurophil granule lumen / Separation of Sister Chromatids / Regulation of PLK1 Activity at G2/M Transition / positive regulation of cold-induced thermogenesis / cell cortex / microtubule / cell division / Neutrophil degranulation / centrosome / ATP hydrolysis activity / RNA binding / extracellular exosome / extracellular region / ATP binding / identical protein binding / membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
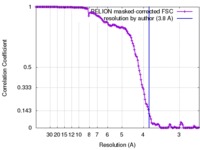
| Method | single particle reconstruction / cryo EM / Resolution: 3.8 Å | |||||||||
 Authors Authors | Zhang K / Foster H | |||||||||
| Funding support |  United Kingdom, 2 items United Kingdom, 2 items
| |||||||||
 Citation Citation |  Journal: Cell / Year: 2017 Journal: Cell / Year: 2017Title: Cryo-EM Reveals How Human Cytoplasmic Dynein Is Auto-inhibited and Activated. Authors: Kai Zhang / Helen E Foster / Arnaud Rondelet / Samuel E Lacey / Nadia Bahi-Buisson / Alexander W Bird / Andrew P Carter /    Abstract: Cytoplasmic dynein-1 binds dynactin and cargo adaptor proteins to form a transport machine capable of long-distance processive movement along microtubules. However, it is unclear why dynein-1 moves ...Cytoplasmic dynein-1 binds dynactin and cargo adaptor proteins to form a transport machine capable of long-distance processive movement along microtubules. However, it is unclear why dynein-1 moves poorly on its own or how it is activated by dynactin. Here, we present a cryoelectron microscopy structure of the complete 1.4-megadalton human dynein-1 complex in an inhibited state known as the phi-particle. We reveal the 3D structure of the cargo binding dynein tail and show how self-dimerization of the motor domains locks them in a conformation with low microtubule affinity. Disrupting motor dimerization with structure-based mutagenesis drives dynein-1 into an open form with higher affinity for both microtubules and dynactin. We find the open form is also inhibited for movement and that dynactin relieves this by reorienting the motor domains to interact correctly with microtubules. Our model explains how dynactin binding to the dynein-1 tail directly stimulates its motor activity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_3698.map.gz emd_3698.map.gz | 15.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-3698-v30.xml emd-3698-v30.xml emd-3698.xml emd-3698.xml | 20.1 KB 20.1 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_3698_fsc.xml emd_3698_fsc.xml | 13.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_3698.png emd_3698.png | 125.4 KB | ||
| Filedesc metadata |  emd-3698.cif.gz emd-3698.cif.gz | 9.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-3698 http://ftp.pdbj.org/pub/emdb/structures/EMD-3698 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3698 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-3698 | HTTPS FTP |
-Validation report
| Summary document |  emd_3698_validation.pdf.gz emd_3698_validation.pdf.gz | 236.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_3698_full_validation.pdf.gz emd_3698_full_validation.pdf.gz | 235.4 KB | Display | |
| Data in XML |  emd_3698_validation.xml.gz emd_3698_validation.xml.gz | 13.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3698 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3698 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3698 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-3698 | HTTPS FTP |
-Related structure data
| Related structure data |  5nugMC  3703C  3704C  3705C  3706C  3707C  5nvsC  5nvuC  5nw4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_3698.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_3698.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | locally masked region of motor domain from complete human dynein-1 phi-particle | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.32 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Motor domains from human cytoplasmic dynein-1 in the phi-particle...
| Entire | Name: Motor domains from human cytoplasmic dynein-1 in the phi-particle conformation |
|---|---|
| Components |
|
-Supramolecule #1: Motor domains from human cytoplasmic dynein-1 in the phi-particle...
| Supramolecule | Name: Motor domains from human cytoplasmic dynein-1 in the phi-particle conformation type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Cytoplasmic dynein 1 heavy chain 1
| Macromolecule | Name: Cytoplasmic dynein 1 heavy chain 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 533.08325 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSEPGGGGGE DGSAGLEVSA VQNVADVSVL QKHLRKLVPL LLEDGGEAPA ALEAALEEKS ALEQMRKFLS DPQVHTVLVE RSTLKEDVG DEGEEEKEFI SYNINIDIHY GVKSNSLAFI KRTPVIDADK PVSSQLRVLT LSEDSPYETL HSFISNAVAP F FKSYIRES ...String: MSEPGGGGGE DGSAGLEVSA VQNVADVSVL QKHLRKLVPL LLEDGGEAPA ALEAALEEKS ALEQMRKFLS DPQVHTVLVE RSTLKEDVG DEGEEEKEFI SYNINIDIHY GVKSNSLAFI KRTPVIDADK PVSSQLRVLT LSEDSPYETL HSFISNAVAP F FKSYIRES GKADRDGDKM APSVEKKIAE LEMGLLHLQQ NIEIPEISLP IHPMITNVAK QCYERGEKPK VTDFGDKVED PT FLNQLQS GVNRWIREIQ KVTKLDRDPA SGTALQEISF WLNLERALYR IQEKRESPEV LLTLDILKHG KRFHATVSFD TDT GLKQAL ETVNDYNPLM KDFPLNDLLS ATELDKIRQA LVAIFTHLRK IRNTKYPIQR ALRLVEAISR DLSSQLLKVL GTRK LMHVA YEEFEKVMVA CFEVFQTWDD EYEKLQVLLR DIVKRKREEN LKMVWRINPA HRKLQARLDQ MRKFRRQHEQ LRAVI VRVL RPQVTAVAQQ NQGEVPEPQD MKVAEVLFDA ADANAIEEVN LAYENVKEVD GLDVSKEGTE AWEAAMKRYD ERIDRV ETR ITARLRDQLG TAKNANEMFR IFSRFNALFV RPHIRGAIRE YQTQLIQRVK DDIESLHDKF KVQYPQSQAC KMSHVRD LP PVSGSIIWAK QIDRQLTAYM KRVEDVLGKG WENHVEGQKL KQDGDSFRMK LNTQEIFDDW ARKVQQRNLG VSGRIFTI E STRVRGRTGN VLKLKVNFLP EIITLSKEVR NLKWLGFRVP LAIVNKAHQA NQLYPFAISL IESVRTYERT CEKVEERNT ISLLVAGLKK EVQALIAEGI ALVWESYKLD PYVQRLAETV FNFQEKVDDL LIIEEKIDLE VRSLETCMYD HKTFSEILNR VQKAVDDLN LHSYSNLPIW VNKLDMEIER ILGVRLQAGL RAWTQVLLGQ AEDKAEVDMD TDAPQVSHKP GGEPKIKNVV H ELRITNQV IYLNPPIEEC RYKLYQEMFA WKMVVLSLPR IQSQRYQVGV HYELTEEEKF YRNALTRMPD GPVALEESYS AV MGIVSEV EQYVKVWLQY QCLWDMQAEN IYNRLGEDLN KWQALLVQIR KARGTFDNAE TKKEFGPVVI DYGKVQSKVN LKY DSWHKE VLSKFGQMLG SNMTEFHSQI SKSRQELEQH SVDTASTSDA VTFITYVQSL KRKIKQFEKQ VELYRNGQRL LEKQ RFQFP PSWLYIDNIE GEWGAFNDIM RRKDSAIQQQ VANLQMKIVQ EDRAVESRTT DLLTDWEKTK PVTGNLRPEE ALQAL TIYE GKFGRLKDDR EKCAKAKEAL ELTDTGLLSG SEERVQVALE ELQDLKGVWS ELSKVWEQID QMKEQPWVSV QPRKLR QNL DALLNQLKSF PARLRQYASY EFVQRLLKGY MKINMLVIEL KSEALKDRHW KQLMKRLHVN WVVSELTLGQ IWDVDLQ KN EAIVKDVLLV AQGEMALEEF LKQIREVWNT YELDLVNYQN KCRLIRGWDD LFNKVKEHIN SVSAMKLSPY YKVFEEDA L SWEDKLNRIM ALFDVWIDVQ RRWVYLEGIF TGSADIKHLL PVETQRFQSI STEFLALMKK VSKSPLVMDV LNIQGVQRS LERLADLLGK IQKALGEYLE RERSSFPRFY FVGDEDLLEI IGNSKNVAKL QKHFKKMFAG VSSIILNEDN SVVLGISSRE GEEVMFKTP VSITEHPKIN EWLTLVEKEM RVTLAKLLAE SVTEVEIFGK ATSIDPNTYI TWIDKYQAQL VVLSAQIAWS E NVETALSS MGGGGDAAPL HSVLSNVEVT LNVLADSVLM EQPPLRRRKL EHLITELVHQ RDVTRSLIKS KIDNAKSFEW LS QMRFYFD PKQTDVLQQL SIQMANAKFN YGFEYLGVQD KLVQTPLTDR CYLTMTQALE ARLGGSPFGP AGTGKTESVK ALG HQLGRF VLVFNCDETF DFQAMGRIFV GLCQVGAWGC FDEFNRLEER MLSAVSQQVQ CIQEALREHS NPNYDKTSAP ITCE LLNKQ VKVSPDMAIF ITMNPGYAGR SNLPDNLKKL FRSLAMTKPD RQLIAQVMLY SQGFRTAEVL ANKIVPFFKL CDEQL SSQS HYDFGLRALK SVLVSAGNVK RERIQKIKRE KEERGEAVDE GEIAENLPEQ EILIQSVCET MVPKLVAEDI PLLFSL LSD VFPGVQYHRG EMTALREELK KVCQEMYLTY GDGEEVGGMW VEKVLQLYQI TQINHGLMMV GPSGSGKSMA WRVLLKA LE RLEGVEGVAH IIDPKAISKD HLYGTLDPNT REWTDGLFTH VLRKIIDSVR GELQKRQWIV FDGDVDPEWV ENLNSVLD D NKLLTLPNGE RLSLPPNVRI MFEVQDLKYA TLATVSRCGM VWFSEDVLST DMIFNNFLAR LRSIPLDEGE DEAQRRRKG KEDEGEEAAS PMLQIQRDAA TIMQPYFTSN GLVTKALEHA FQLEHIMDLT RLRCLGSLFS MLHQACRNVA QYNANHPDFP MQIEQLERY IQRYLVYAIL WSLSGDSRLK MRAELGEYIR RITTVPLPTA PNIPIIDYEV SISGEWSPWQ AKVPQIEVET H KVAAPDVV VPTLDTVRHE ALLYTWLAEH KPLVLCGPPG SGKTMTLFSA LRALPDMEVV GLNFSSATTP ELLLKTFDHY CE YRRTPNG VVLAPVQLGK WLVLFCDEIN LPDMDKYGTQ RVISFIRQMV EHGGFYRTSD QTWVKLERIQ FVGACNPPTD PGR KPLSHR FLRHVPVVYV DYPGPASLTQ IYGTFNRAML RLIPSLRTYA EPLTAAMVEF YTMSQERFTQ DTQPHYIYSP REMT RWVRG IFEALRPLET LPVEGLIRIW AHEALRLFQD RLVEDEERRW TDENIDTVAL KHFPNIDREK AMSRPILYSN WLSKD YIPV DQEELRDYVK ARLKVFYEEE LDVPLVLFNE VLDHVLRIDR IFRQPQGHLL LIGVSGAGKT TLSRFVAWMN GLSVYQ IKV HRKYTGEDFD EDLRTVLRRS GCKNEKIAFI MDESNVLDSG FLERMNTLLA NGEVPGLFEG DEYATLMTQC KEGAQKE GL MLDSHEELYK WFTSQVIRNL HVVFTMNPSS EGLKDRAATS PALFNRCVLN WFGDWSTEAL YQVGKEFTSK MDLEKPNY I VPDYMPVVYD KLPQPPSHRE AIVNSCVFVH QTLHQANARL AKRGGRTMAI TPRHYLDFIN HYANLFHEKR SELEEQQMH LNVGLRKIKE TVDQVEELRR DLRIKSQELE VKNAAANDKL KKMVKDQQEA EKKKVMSQEI QEQLHKQQEV IADKQMSVKE DLDKVEPAV IEAQNAVKSI KKQHLVEVRS MANPPAAVKL ALESICLLLG ESTTDWKQIR SIIMRENFIP TIVNFSAEEI S DAIREKMK KNYMSNPSYN YEIVNRASLA CGPMVKWAIA QLNYADMLKR VEPLRNELQK LEDDAKDNQQ KANEVEQMIR DL EASIARY KEEYAVLISE AQAIKADLAA VEAKVNRSTA LLKSLSAERE RWEKTSETFK NQMSTIAGDC LLSAAFIAYA GYF DQQMRQ NLFTTWSHHL QQANIQFRTD IARTEYLSNA DERLRWQASS LPADDLCTEN AIMLKRFNRY PLIIDPSGQA TEFI MNEYK DRKITRTSFL DDAFRKNLES ALRFGNPLLV QDVESYDPVL NPVLNREVRR TGGRVLITLG DQDIDLSPSF VIFLS TRDP TVEFPPDLCS RVTFVNFTVT RSSLQSQCLN EVLKAERPDV DEKRSDLLKL QGEFQLRLRQ LEKSLLQALN EVKGRI LDD DTIITTLENL KREAAEVTRK VEETDIVMQE VETVSQQYLP LSTACSSIYF TMESLKQIHF LYQYSLQFFL DIYHNVL YE NPNLKGVTDH TQRLSIITKD LFQVAFNRVA RGMLHQDHIT FAMLLARIKL KGTVGEPTYD AEFQHFLRGN EIVLSAGS T PRIQGLTVEQ AEAVVRLSCL PAFKDLIAKV QADEQFGIWL DSSSPEQTVP YLWSEETPAT PIGQAIHRLL LIQAFRPDR LLAMAHMFVS TNLGESFMSI MEQPLDLTHI VGTEVKPNTP VLMCSVPGYD ASGHVEDLAA EQNTQITSIA IGSAEGFNQA DKAINTAVK SGRWVMLKNV HLAPGWLMQL EKKLHSLQPH ACFRLFLTME INPKVPVNLL RAGRIFVFEP PPGVKANMLR T FSSIPVSR ICKSPNERAR LYFLLAWFHA IIQERLRYAP LGWSKKYEFG ESDLRSACDT VDTWLDDTAK GRQNISPDKI PW SALKTLM AQSIYGGRVD NEFDQRLLNT FLERLFTTRS FDSEFKLACK VDGHKDIQMP DGIRREEFVQ WVELLPDTQT PSW LGLPNN AERVLLTTQG VDMISKMLKM QMLEDEDDLA YAETEKKTRT DSTSDGRPAW MRTLHTTASN WLHLIPQTLS HLKR TVENI KDPLFRFFER EVKMGAKLLQ DVRQDLADVV QVCEGKKKQT NYLRTLINEL VKGILPRSWS HYTVPAGMTV IQWVS DFSE RIKQLQNISL AAASGGAKEL KNIHVCLGGL FVPEAYITAT RQYVAQANSW SLEELCLEVN VTTSQGATLD ACSFGV TGL KLQGATCNNN KLSLSNAIST ALPLTQLRWV KQTNTEKKAS VVTLPVYLNF TRADLIFTVD FEIATKEDPR SFYERGV AV LCTE UniProtKB: Cytoplasmic dynein 1 heavy chain 1 |
-Macromolecule #2: ADENOSINE-5'-DIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-DIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 6 / Formula: ADP |
|---|---|
| Molecular weight | Theoretical: 427.201 Da |
| Chemical component information |  ChemComp-ADP: |
-Macromolecule #3: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 3 / Number of copies: 2 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Macromolecule #4: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 4 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL |
|---|---|
| Buffer | pH: 7.4 Details: 20mM HEPES pH7.4, 150 mM KCl, 1mM MgCl2, 5 mM DTT, 0.1 mM ATP |
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: CONTINUOUS / Support film - Film thickness: 50 / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 25 sec. / Pretreatment - Atmosphere: OTHER |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
| Details | vitrification was performed immediately after gel filtration. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 90.0 K / Max: 100.0 K |
| Image recording | Film or detector model: FEI FALCON II (4k x 4k) / Detector mode: INTEGRATING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Digitization - Frames/image: 3-34 / Number grids imaged: 6746 / Number real images: 312198 / Average exposure time: 2.0 sec. / Average electron dose: 1.6 e/Å2 Details: Images were also collected using K2 summit detetor in counting mode |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Calibrated defocus max: 5.0 µm / Calibrated defocus min: 1.5 µm / Calibrated magnification: 106061 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 5.0 µm / Nominal defocus min: 1.5 µm / Nominal magnification: 59000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: RECIPROCAL / Protocol: AB INITIO MODEL / Overall B value: 145 |
|---|---|
| Output model |  PDB-5nug: |
 Movie
Movie Controller
Controller






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)