[English] 日本語
 Yorodumi
Yorodumi- EMDB-21168: BG505-SOSIP-T33_dn2A nanoparticle fusion component in complex wit... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21168 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
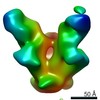
| Title | BG505-SOSIP-T33_dn2A nanoparticle fusion component in complex with VRC01-Fab | |||||||||
 Map data Map data | BG505-SOSIP-T33_dn2A fusion component in complex with VRC01-Fab - Negative Stain Map | |||||||||
 Sample Sample |
| |||||||||
| Biological species | synthetic construct (others) | |||||||||
| Method | single particle reconstruction / negative staining / Resolution: 15.62 Å | |||||||||
 Authors Authors | Ward AB / Antanasijevic A | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2020 Journal: Elife / Year: 2020Title: Tailored design of protein nanoparticle scaffolds for multivalent presentation of viral glycoprotein antigens. Authors: George Ueda / Aleksandar Antanasijevic / Jorge A Fallas / William Sheffler / Jeffrey Copps / Daniel Ellis / Geoffrey B Hutchinson / Adam Moyer / Anila Yasmeen / Yaroslav Tsybovsky / Young- ...Authors: George Ueda / Aleksandar Antanasijevic / Jorge A Fallas / William Sheffler / Jeffrey Copps / Daniel Ellis / Geoffrey B Hutchinson / Adam Moyer / Anila Yasmeen / Yaroslav Tsybovsky / Young-Jun Park / Matthew J Bick / Banumathi Sankaran / Rebecca A Gillespie / Philip Jm Brouwer / Peter H Zwart / David Veesler / Masaru Kanekiyo / Barney S Graham / Rogier W Sanders / John P Moore / Per Johan Klasse / Andrew B Ward / Neil P King / David Baker /   Abstract: Multivalent presentation of viral glycoproteins can substantially increase the elicitation of antigen-specific antibodies. To enable a new generation of anti-viral vaccines, we designed self- ...Multivalent presentation of viral glycoproteins can substantially increase the elicitation of antigen-specific antibodies. To enable a new generation of anti-viral vaccines, we designed self-assembling protein nanoparticles with geometries tailored to present the ectodomains of influenza, HIV, and RSV viral glycoprotein trimers. We first designed trimers tailored for antigen fusion, featuring N-terminal helices positioned to match the C termini of the viral glycoproteins. Trimers that experimentally adopted their designed configurations were incorporated as components of tetrahedral, octahedral, and icosahedral nanoparticles, which were characterized by cryo-electron microscopy and assessed for their ability to present viral glycoproteins. Electron microscopy and antibody binding experiments demonstrated that the designed nanoparticles presented antigenically intact prefusion HIV-1 Env, influenza hemagglutinin, and RSV F trimers in the predicted geometries. This work demonstrates that antigen-displaying protein nanoparticles can be designed from scratch, and provides a systematic way to investigate the influence of antigen presentation geometry on the immune response to vaccination. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21168.map.gz emd_21168.map.gz | 11.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21168-v30.xml emd-21168-v30.xml emd-21168.xml emd-21168.xml | 13.6 KB 13.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_21168.png emd_21168.png | 43.9 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21168 http://ftp.pdbj.org/pub/emdb/structures/EMD-21168 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21168 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21168 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_21168.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21168.map.gz / Format: CCP4 / Size: 15.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | BG505-SOSIP-T33_dn2A fusion component in complex with VRC01-Fab - Negative Stain Map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 2.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : BG505-SOSIP-T33_dn2A Nanoparticle Fusion Construct in complex wit...
| Entire | Name: BG505-SOSIP-T33_dn2A Nanoparticle Fusion Construct in complex with VRC01-Fab |
|---|---|
| Components |
|
-Supramolecule #1: BG505-SOSIP-T33_dn2A Nanoparticle Fusion Construct in complex wit...
| Supramolecule | Name: BG505-SOSIP-T33_dn2A Nanoparticle Fusion Construct in complex with VRC01-Fab type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Nanoparticle Fusion Construct in complex with 3 copies of VRC01 Fab Fragment |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant strain: HEK293F / Recombinant plasmid: pPPI4 Homo sapiens (human) / Recombinant strain: HEK293F / Recombinant plasmid: pPPI4 |
-Macromolecule #1: BG505-SOSIP-T33_dn2A nanoparticle fusion component
| Macromolecule | Name: BG505-SOSIP-T33_dn2A nanoparticle fusion component / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MKRGLCCVLL LCGAVFVSPS QEIHARFRRG ARAENLWVTV YYGVPVWKDA ETTLFCASDA KAYETKKHNV WATHCCVPTD PNPQEIHLEN VTEEFNMWKN NMVEQMHTDI ISLWDQSLKP CVKLTPLCVT LQCTNVTNNI TDDMRGELKN CSFNMTTELR DKKQKVYSLF ...String: MKRGLCCVLL LCGAVFVSPS QEIHARFRRG ARAENLWVTV YYGVPVWKDA ETTLFCASDA KAYETKKHNV WATHCCVPTD PNPQEIHLEN VTEEFNMWKN NMVEQMHTDI ISLWDQSLKP CVKLTPLCVT LQCTNVTNNI TDDMRGELKN CSFNMTTELR DKKQKVYSLF YRLDVVQINE NQGNRSNNSN KEYRLINCNT SAITQACPKV SFEPIPIHYC APAGFAILKC KDKKFNGTGP CTNVSTVQCT HGIKPVVSTQ LLLNGSLAEE EVIIRSENIT NNAKNILVQL NESVQINCTR PNNNTVKSIR IGPGQWFYYT GDIIGDIRQA HCNVSKATWN ETLGKVVKQL RKHFGNNTII RFANSSGGDL EVTTHSFNCG GEFFYCNTSG LFNSTWISNT SVQGSNSTGS NDSITLPCRI KQIINMWQRI GQAMYAPPIQ GVIRCVSNIT GLILTRDGGS TNSTTETFRP GGGDMRDNWR SELYKYKVVK IEPLGVAPTR CKRRVVGRRR RRRAVGIGAV SLGFLGAAGS TMGAASMTLT VQARNLLSGI VQQQSNLLRA PECQQHLLKD THWGIKQLQA RVLAVEHYLR DQQLLGIWGC SGKLICCTNV PWNSSWSNRN LSEIWDNMTW LQWDKEISNY TQIIYGLLEE SQNQQEKNEQ DLLELDKWAS LWGSMGNLAE KMYKAGNAMY RKGQYTIAII AYTLALLKDP NNAEAWYNLG NAAYKKGEYD EAIEAYQKAL ELDPNNAEAW YNLGNAYYKQ GDYDEAIEYY KKALRLDPRN VDAIENLIEA EEKQGAS |
-Experimental details
-Structure determination
| Method | negative staining |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.02 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: TBS buffer, pH 7.4 | |||||||||
| Staining | Type: NEGATIVE / Material: Uranyl Formate Details: Sample diluted to 0.02 mg/mL. 3 uL was applied onto the grid, blotted off, and then stained with 2% uranyl formate for 60 seconds. | |||||||||
| Grid | Model: Homemade / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: CONTINUOUS | |||||||||
| Details | BG505-SOSIP-T33_dn2A and VRC01 Fab samples purified by SEC. BG505-SOSIP-T33_dn2A was incubated with 6-fold molar excess of VRC01-Fab for 1 hour, diluted to 0.02mg/ml and imaged. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TECNAI SPIRIT |
|---|---|
| Image recording | Film or detector model: TVIPS TEMCAM-F416 (4k x 4k) / Average electron dose: 25.0 e/Å2 |
| Electron beam | Acceleration voltage: 120 kV / Electron source: LAB6 |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 1.5 µm |
| Experimental equipment |  Model: Tecnai Spirit / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


 UCSF Chimera
UCSF Chimera






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)





















