+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-21172 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | De novo designed tetrahedral nanoparticle T33_dn10 | |||||||||
 Map data Map data | T33_dn10 Nanoparticle Map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | De novo / Nanoparticle / DE NOVO PROTEIN | |||||||||
| Biological species | synthetic construct (others) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.86 Å | |||||||||
 Authors Authors | Antanasijevic A / Ward AB | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Elife / Year: 2020 Journal: Elife / Year: 2020Title: Tailored design of protein nanoparticle scaffolds for multivalent presentation of viral glycoprotein antigens. Authors: George Ueda / Aleksandar Antanasijevic / Jorge A Fallas / William Sheffler / Jeffrey Copps / Daniel Ellis / Geoffrey B Hutchinson / Adam Moyer / Anila Yasmeen / Yaroslav Tsybovsky / Young- ...Authors: George Ueda / Aleksandar Antanasijevic / Jorge A Fallas / William Sheffler / Jeffrey Copps / Daniel Ellis / Geoffrey B Hutchinson / Adam Moyer / Anila Yasmeen / Yaroslav Tsybovsky / Young-Jun Park / Matthew J Bick / Banumathi Sankaran / Rebecca A Gillespie / Philip Jm Brouwer / Peter H Zwart / David Veesler / Masaru Kanekiyo / Barney S Graham / Rogier W Sanders / John P Moore / Per Johan Klasse / Andrew B Ward / Neil P King / David Baker /   Abstract: Multivalent presentation of viral glycoproteins can substantially increase the elicitation of antigen-specific antibodies. To enable a new generation of anti-viral vaccines, we designed self- ...Multivalent presentation of viral glycoproteins can substantially increase the elicitation of antigen-specific antibodies. To enable a new generation of anti-viral vaccines, we designed self-assembling protein nanoparticles with geometries tailored to present the ectodomains of influenza, HIV, and RSV viral glycoprotein trimers. We first designed trimers tailored for antigen fusion, featuring N-terminal helices positioned to match the C termini of the viral glycoproteins. Trimers that experimentally adopted their designed configurations were incorporated as components of tetrahedral, octahedral, and icosahedral nanoparticles, which were characterized by cryo-electron microscopy and assessed for their ability to present viral glycoproteins. Electron microscopy and antibody binding experiments demonstrated that the designed nanoparticles presented antigenically intact prefusion HIV-1 Env, influenza hemagglutinin, and RSV F trimers in the predicted geometries. This work demonstrates that antigen-displaying protein nanoparticles can be designed from scratch, and provides a systematic way to investigate the influence of antigen presentation geometry on the immune response to vaccination. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_21172.map.gz emd_21172.map.gz | 96.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-21172-v30.xml emd-21172-v30.xml emd-21172.xml emd-21172.xml | 20.6 KB 20.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_21172_fsc.xml emd_21172_fsc.xml | 10.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_21172.png emd_21172.png | 190.6 KB | ||
| Masks |  emd_21172_msk_1.map emd_21172_msk_1.map | 103 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-21172.cif.gz emd-21172.cif.gz | 6.2 KB | ||
| Others |  emd_21172_half_map_1.map.gz emd_21172_half_map_1.map.gz emd_21172_half_map_2.map.gz emd_21172_half_map_2.map.gz | 80.7 MB 80.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-21172 http://ftp.pdbj.org/pub/emdb/structures/EMD-21172 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21172 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-21172 | HTTPS FTP |
-Related structure data
| Related structure data |  6vfhMC  6v8eC  6vehC  6vfiC  6vfjC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_21172.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_21172.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | T33_dn10 Nanoparticle Map | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
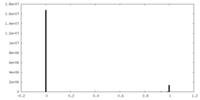
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_21172_msk_1.map emd_21172_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
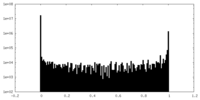
| Density Histograms |
-Half map: T33 dn10 Nanoparticle - Halfmap 1
| File | emd_21172_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | T33_dn10 Nanoparticle - Halfmap 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
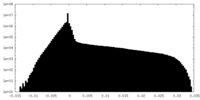
| Density Histograms |
-Half map: T33 dn10 Nanoparticle - Halfmap 2
| File | emd_21172_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | T33_dn10 Nanoparticle - Halfmap 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
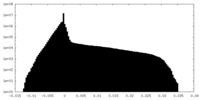
| Density Histograms |
- Sample components
Sample components
-Entire : De novo designed tetrahedral nanoparticle T33_dn10
| Entire | Name: De novo designed tetrahedral nanoparticle T33_dn10 |
|---|---|
| Components |
|
-Supramolecule #1: De novo designed tetrahedral nanoparticle T33_dn10
| Supramolecule | Name: De novo designed tetrahedral nanoparticle T33_dn10 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Self-assembling nanoparticle with tetrahedral symmetry |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 546 KDa |
-Macromolecule #1: T33_dn10A
| Macromolecule | Name: T33_dn10A / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 14.082408 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGEEAELAYL LGELAYKLGE YRIAIRAYRI ALKRDPNNAE AWYNLGNAYY KQGDYDEAIE YYQKALELDP NNAEAWYNLG NAYYKQGDY DEAIEYYEKA LELDPENLEA LQNLLNAMDK QG |
-Macromolecule #2: T33_dn10B
| Macromolecule | Name: T33_dn10B / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 31.46683 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MIEEVVAEMI DILAESSKKS IEELARAADN KTTEKAVAEA IEEIARLATA AIQLIEALAK NLASEEFMAR AISAIAELAK KAIEAIYRL ADNHTTDTFM ARAIAAIANL AVTAILAIAA LASNHTTEEF MARAISAIAE LAKKAIEAIY RLADNHTTDK F MAAAIEAI ...String: MIEEVVAEMI DILAESSKKS IEELARAADN KTTEKAVAEA IEEIARLATA AIQLIEALAK NLASEEFMAR AISAIAELAK KAIEAIYRL ADNHTTDTFM ARAIAAIANL AVTAILAIAA LASNHTTEEF MARAISAIAE LAKKAIEAIY RLADNHTTDK F MAAAIEAI ALLATLAILA IALLASNHTT EKFMARAIMA IAILAAKAIE AIYRLADNHT SPTYIEKAIE AIEKIARKAI KA IEMLAKN ITTEEYKEKA KKIIDIIRKL AKMAIKKLED NRTLEHHHHH H |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4.2 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.4 Component:
Details: TBS buffer, 0.2um filtered, 0.06mM DDM detergent added immediately before freezing | |||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 11 / Pretreatment - Type: PLASMA CLEANING / Pretreatment - Time: 10 sec. / Pretreatment - Atmosphere: OTHER | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK IV Details: 0.06mM DDM detergent (from an 8X stock) added immediately before freezing. | |||||||||
| Details | Nanoparticles are generated by co-expression of two components (A and B) in E coli. Assembled particles are purified using a combination of Ni-affinity chromatography and gel-filtration chromatography. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 70.0 K / Max: 90.0 K |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Number real images: 502 / Average electron dose: 50.4 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 29000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | Tetrahedral nanoparticle T33_dn10 model was docked into the reconstructed map using UCSF Chimera. The model was then relaxed using a combination of Rosetta relaxed refinement and manual refinement in Coot. |
|---|---|
| Refinement | Space: REAL / Protocol: OTHER |
| Output model |  PDB-6vfh: |
 Movie
Movie Controller
Controller





















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)