[English] 日本語
 Yorodumi
Yorodumi- EMDB-10391: Structure of human Sox11 transcription factor in complex with a n... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10391 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of human Sox11 transcription factor in complex with a nucleosome | ||||||||||||
 Map data Map data | Structure of human Sox11 transcription factor in complex with a nucleosome | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Nucleosome / DNA / histones / Sox11 / transcription factor / pioneer factor / NUCLEAR PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationclosure of optic fissure / positive regulation of lens epithelial cell proliferation / soft palate development / negative regulation of transcription regulatory region DNA binding / cornea development in camera-type eye / noradrenergic neuron differentiation / negative regulation of lymphocyte proliferation / regulation of transforming growth factor beta receptor signaling pathway / lens morphogenesis in camera-type eye / positive regulation of hippo signaling ...closure of optic fissure / positive regulation of lens epithelial cell proliferation / soft palate development / negative regulation of transcription regulatory region DNA binding / cornea development in camera-type eye / noradrenergic neuron differentiation / negative regulation of lymphocyte proliferation / regulation of transforming growth factor beta receptor signaling pathway / lens morphogenesis in camera-type eye / positive regulation of hippo signaling / embryonic skeletal system morphogenesis / neuroepithelial cell differentiation / embryonic digestive tract morphogenesis / camera-type eye morphogenesis / sympathetic nervous system development / positive regulation of BMP signaling pathway / positive regulation of hormone secretion / hard palate development / oligodendrocyte development / positive regulation of ossification / positive regulation of neurogenesis / negative regulation of glial cell proliferation / ventricular septum morphogenesis / positive regulation of stem cell proliferation / spinal cord development / eyelid development in camera-type eye / lung morphogenesis / outflow tract morphogenesis / skeletal muscle cell differentiation / positive regulation of osteoblast differentiation / glial cell proliferation / negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / CENP-A containing nucleosome / Packaging Of Telomere Ends / positive regulation of neuron differentiation / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / telomere organization / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / Interleukin-7 signaling / RNA Polymerase I Promoter Opening / Inhibition of DNA recombination at telomere / Assembly of the ORC complex at the origin of replication / Meiotic synapsis / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / Regulation of endogenous retroelements by KRAB-ZFP proteins / innate immune response in mucosa / Defective pyroptosis / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / kidney development / RNA Polymerase I Promoter Escape / Nonhomologous End-Joining (NHEJ) / Transcriptional regulation by small RNAs / Formation of the beta-catenin:TCF transactivating complex / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / HDMs demethylate histones / brain development / G2/M DNA damage checkpoint / NoRC negatively regulates rRNA expression / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / DNA Damage/Telomere Stress Induced Senescence / Pre-NOTCH Transcription and Translation / Meiotic recombination / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Metalloprotease DUBs / RMTs methylate histone arginines / Transcriptional regulation of granulopoiesis / neuron differentiation / HCMV Early Events / antimicrobial humoral immune response mediated by antimicrobial peptide / structural constituent of chromatin / UCH proteinases / antibacterial humoral response / heterochromatin formation / nucleosome / nervous system development / nucleosome assembly / E3 ubiquitin ligases ubiquitinate target proteins / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / HATs acetylate histones / RUNX1 regulates transcription of genes involved in differentiation of HSCs / Factors involved in megakaryocyte development and platelet production / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / chromatin organization Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | ||||||||||||
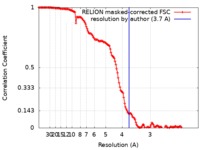
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | ||||||||||||
 Authors Authors | Dodonova SO / Zhu F | ||||||||||||
| Funding support |  Germany, 3 items Germany, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: Nucleosome-bound SOX2 and SOX11 structures elucidate pioneer factor function. Authors: Svetlana O Dodonova / Fangjie Zhu / Christian Dienemann / Jussi Taipale / Patrick Cramer /   Abstract: 'Pioneer' transcription factors are required for stem-cell pluripotency, cell differentiation and cell reprogramming. Pioneer factors can bind nucleosomal DNA to enable gene expression from regions ...'Pioneer' transcription factors are required for stem-cell pluripotency, cell differentiation and cell reprogramming. Pioneer factors can bind nucleosomal DNA to enable gene expression from regions of the genome with closed chromatin. SOX2 is a prominent pioneer factor that is essential for pluripotency and self-renewal of embryonic stem cells. Here we report cryo-electron microscopy structures of the DNA-binding domains of SOX2 and its close homologue SOX11 bound to nucleosomes. The structures show that SOX factors can bind and locally distort DNA at superhelical location 2. The factors also facilitate detachment of terminal nucleosomal DNA from the histone octamer, which increases DNA accessibility. SOX-factor binding to the nucleosome can also lead to a repositioning of the N-terminal tail of histone H4 that includes residue lysine 16. We speculate that this repositioning is incompatible with higher-order nucleosome stacking, which involves contacts of the H4 tail with a neighbouring nucleosome. Our results indicate that pioneer transcription factors can use binding energy to initiate chromatin opening, and thereby facilitate nucleosome remodelling and subsequent transcription. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10391.map.gz emd_10391.map.gz | 7.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10391-v30.xml emd-10391-v30.xml emd-10391.xml emd-10391.xml | 27 KB 27 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10391_fsc.xml emd_10391_fsc.xml | 14.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_10391.png emd_10391.png | 145.6 KB | ||
| Filedesc metadata |  emd-10391.cif.gz emd-10391.cif.gz | 7.5 KB | ||
| Others |  emd_10391_half_map_1.map.gz emd_10391_half_map_1.map.gz emd_10391_half_map_2.map.gz emd_10391_half_map_2.map.gz | 9.7 MB 9.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10391 http://ftp.pdbj.org/pub/emdb/structures/EMD-10391 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10391 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10391 | HTTPS FTP |
-Validation report
| Summary document |  emd_10391_validation.pdf.gz emd_10391_validation.pdf.gz | 742.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_10391_full_validation.pdf.gz emd_10391_full_validation.pdf.gz | 741.9 KB | Display | |
| Data in XML |  emd_10391_validation.xml.gz emd_10391_validation.xml.gz | 15.1 KB | Display | |
| Data in CIF |  emd_10391_validation.cif.gz emd_10391_validation.cif.gz | 20.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10391 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10391 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10391 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10391 | HTTPS FTP |
-Related structure data
| Related structure data |  6t7aMC 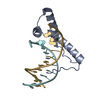 6t78C  6t79C  6t7bC  6t7cC  6t7dC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10391.map.gz / Format: CCP4 / Size: 10.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10391.map.gz / Format: CCP4 / Size: 10.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of human Sox11 transcription factor in complex with a nucleosome | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: half map 1
| File | emd_10391_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half map 2
| File | emd_10391_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Structure of human Sox11 transcription factor in complex with a n...
+Supramolecule #1: Structure of human Sox11 transcription factor in complex with a n...
+Supramolecule #2: Sox11 transcription factor, histones
+Supramolecule #3: DNA
+Macromolecule #1: Histone H3.2
+Macromolecule #2: Histone H4
+Macromolecule #3: Histone H2A type 1-B/E
+Macromolecule #4: Histone H2B type 1-K
+Macromolecule #7: Transcription factor SOX-11
+Macromolecule #5: DNA (147-MER)
+Macromolecule #6: DNA (147-MER)
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 120 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa / Details: 0.39 mB, 25 mA | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 289 K / Instrument: FEI VITROBOT MARK IV Details: The sample was applied onto glow-discharged Quantifoil holey carbon grids. The grids were blotted from both sides for 5-10 seconds at 16*C in a chamber at 100% humidity and plunge-frozen ...Details: The sample was applied onto glow-discharged Quantifoil holey carbon grids. The grids were blotted from both sides for 5-10 seconds at 16*C in a chamber at 100% humidity and plunge-frozen into liquid ethane using a manual plunger.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 30 eV |
| Details | At least 50% of the data were collected at 25* stage tilt in order to partially compensate for preferred orientation of particles on the grid, and to improve angular distribution. |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Average electron dose: 1.125 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller




























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)