+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10390 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of a human nucleosome at 3.2 A resolution | ||||||||||||
 Map data Map data | Structure of a human nucleosome at 3.2A | ||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | Nucleosome / DNA / histones / NUCLEAR PROTEIN | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / CENP-A containing nucleosome / Packaging Of Telomere Ends / Negative Regulation of CDH1 Gene Transcription / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere ...negative regulation of megakaryocyte differentiation / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / CENP-A containing nucleosome / Packaging Of Telomere Ends / Negative Regulation of CDH1 Gene Transcription / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / telomere organization / Interleukin-7 signaling / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / RNA Polymerase I Promoter Opening / Inhibition of DNA recombination at telomere / Assembly of the ORC complex at the origin of replication / Meiotic synapsis / SUMOylation of chromatin organization proteins / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / DNA methylation / Condensation of Prophase Chromosomes / Chromatin modifications during the maternal to zygotic transition (MZT) / SIRT1 negatively regulates rRNA expression / HCMV Late Events / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / PRC2 methylates histones and DNA / Regulation of endogenous retroelements by KRAB-ZFP proteins / innate immune response in mucosa / Defective pyroptosis / HDACs deacetylate histones / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / RNA Polymerase I Promoter Escape / Nonhomologous End-Joining (NHEJ) / Transcriptional regulation by small RNAs / Formation of the beta-catenin:TCF transactivating complex / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / HDMs demethylate histones / G2/M DNA damage checkpoint / NoRC negatively regulates rRNA expression / B-WICH complex positively regulates rRNA expression / PKMTs methylate histone lysines / DNA Damage/Telomere Stress Induced Senescence / Pre-NOTCH Transcription and Translation / Meiotic recombination / Activation of anterior HOX genes in hindbrain development during early embryogenesis / Metalloprotease DUBs / Transcriptional regulation of granulopoiesis / RMTs methylate histone arginines / HCMV Early Events / antimicrobial humoral immune response mediated by antimicrobial peptide / structural constituent of chromatin / UCH proteinases / antibacterial humoral response / heterochromatin formation / nucleosome / nucleosome assembly / E3 ubiquitin ligases ubiquitinate target proteins / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / HATs acetylate histones / RUNX1 regulates transcription of genes involved in differentiation of HSCs / Factors involved in megakaryocyte development and platelet production / gene expression / MLL4 and MLL3 complexes regulate expression of PPARG target genes in adipogenesis and hepatic steatosis / chromatin organization / Processing of DNA double-strand break ends / Senescence-Associated Secretory Phenotype (SASP) / Oxidative Stress Induced Senescence / defense response to Gram-negative bacterium / Estrogen-dependent gene expression / killing of cells of another organism / chromosome, telomeric region / defense response to Gram-positive bacterium / Ub-specific processing proteases / Amyloid fiber formation / protein heterodimerization activity / negative regulation of cell population proliferation / chromatin binding / negative regulation of transcription by RNA polymerase II / protein-containing complex / extracellular space / DNA binding / RNA binding / extracellular exosome / extracellular region / nucleoplasm / nucleus / membrane / cytosol Similarity search - Function | ||||||||||||
| Biological species |  Homo sapiens (human) / synthetic construct (others) Homo sapiens (human) / synthetic construct (others) | ||||||||||||
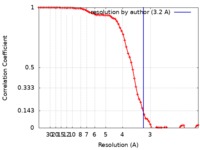
| Method | single particle reconstruction / cryo EM / Resolution: 3.2 Å | ||||||||||||
 Authors Authors | Dodonova SO / Zhu F | ||||||||||||
| Funding support |  Germany, 3 items Germany, 3 items
| ||||||||||||
 Citation Citation |  Journal: Nature / Year: 2020 Journal: Nature / Year: 2020Title: Nucleosome-bound SOX2 and SOX11 structures elucidate pioneer factor function. Authors: Svetlana O Dodonova / Fangjie Zhu / Christian Dienemann / Jussi Taipale / Patrick Cramer /   Abstract: 'Pioneer' transcription factors are required for stem-cell pluripotency, cell differentiation and cell reprogramming. Pioneer factors can bind nucleosomal DNA to enable gene expression from regions ...'Pioneer' transcription factors are required for stem-cell pluripotency, cell differentiation and cell reprogramming. Pioneer factors can bind nucleosomal DNA to enable gene expression from regions of the genome with closed chromatin. SOX2 is a prominent pioneer factor that is essential for pluripotency and self-renewal of embryonic stem cells. Here we report cryo-electron microscopy structures of the DNA-binding domains of SOX2 and its close homologue SOX11 bound to nucleosomes. The structures show that SOX factors can bind and locally distort DNA at superhelical location 2. The factors also facilitate detachment of terminal nucleosomal DNA from the histone octamer, which increases DNA accessibility. SOX-factor binding to the nucleosome can also lead to a repositioning of the N-terminal tail of histone H4 that includes residue lysine 16. We speculate that this repositioning is incompatible with higher-order nucleosome stacking, which involves contacts of the H4 tail with a neighbouring nucleosome. Our results indicate that pioneer transcription factors can use binding energy to initiate chromatin opening, and thereby facilitate nucleosome remodelling and subsequent transcription. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10390.map.gz emd_10390.map.gz | 9.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10390-v30.xml emd-10390-v30.xml emd-10390.xml emd-10390.xml | 25.8 KB 25.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_10390_fsc.xml emd_10390_fsc.xml | 6.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_10390.png emd_10390.png | 172.9 KB | ||
| Filedesc metadata |  emd-10390.cif.gz emd-10390.cif.gz | 7.4 KB | ||
| Others |  emd_10390_half_map_1.map.gz emd_10390_half_map_1.map.gz emd_10390_half_map_2.map.gz emd_10390_half_map_2.map.gz | 9.7 MB 9.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10390 http://ftp.pdbj.org/pub/emdb/structures/EMD-10390 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10390 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10390 | HTTPS FTP |
-Related structure data
| Related structure data |  6t79MC  6t78C  6t7aC  6t7bC  6t7cC  6t7dC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_10390.map.gz / Format: CCP4 / Size: 10.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10390.map.gz / Format: CCP4 / Size: 10.5 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Structure of a human nucleosome at 3.2A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.05 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: half-map 2, nucleosome
| File | emd_10390_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-map 2, nucleosome | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: half-map 1, nucleosome
| File | emd_10390_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | half-map 1, nucleosome | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Nucleosome
| Entire | Name: Nucleosome |
|---|---|
| Components |
|
-Supramolecule #1: Nucleosome
| Supramolecule | Name: Nucleosome / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all / Details: Nucleosome |
|---|---|
| Molecular weight | Theoretical: 205 KDa |
-Supramolecule #2: Proteins
| Supramolecule | Name: Proteins / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#4 / Details: Proteins |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Supramolecule #3: DNA
| Supramolecule | Name: DNA / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #5-#6 / Details: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
-Macromolecule #1: Histone H3.2
| Macromolecule | Name: Histone H3.2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 15.389036 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MARTKQTARK STGGKAPRKQ LATKAARKSA PATGGVKKPH RYRPGTVALR EIRRYQKSTE LLIRKLPFQR LVREIAQDFK TDLRFQSSA VMALQEASEA YLVGLFEDTN LAAIHAKRVT IMPKDIQLAR RIRGERA UniProtKB: Histone H3.2 |
-Macromolecule #2: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.394426 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKGGKG LGKGGAKRHR KVLRDNIQGI TKPAIRRLAR RGGVKRISGL IYEETRGVLK VFLENVIRDA VTYTEHAKRK TVTAMDVVY ALKRQGRTLY GFGG UniProtKB: Histone H4 |
-Macromolecule #3: Histone H2A type 1-B/E
| Macromolecule | Name: Histone H2A type 1-B/E / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 16.707277 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSSHHHHHH ENLYFQSNAP WMSGRGKQGG KARAKAKTRS SRAGLQFPVG RVHRLLRKGN YSERVGAGAP VYLAAVLEYL TAEILELAG NAARDNKKTR IIPRHLQLAI RNDEELNKLL GRVTIAQGGV LPNIQAVLLP KKTESHHKAK GK UniProtKB: Histone H2A type 1-B/E |
-Macromolecule #4: Histone H2B type 1-K
| Macromolecule | Name: Histone H2B type 1-K / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.921213 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPEPAKSAPA PKKGSKKAVT KAQKKDGKKR KRSRKESYSV YVYKVLKQVH PDTGISSKAM GIMNSFVNDI FERIAGEASR LAHYNKRST ITSREIQTAV RLLLPGELAK HAVSEGTKAV TKYTSAK UniProtKB: Histone H2B type 1-K |
-Macromolecule #5: DNA (147-MER)
| Macromolecule | Name: DNA (147-MER) / type: dna / ID: 5 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 45.240848 KDa |
| Sequence | String: (DA)(DT)(DC)(DT)(DA)(DC)(DA)(DC)(DG)(DA) (DC)(DG)(DC)(DT)(DC)(DT)(DT)(DC)(DC)(DG) (DA)(DT)(DC)(DT)(DA)(DA)(DT)(DT)(DT) (DA)(DT)(DG)(DT)(DT)(DT)(DG)(DT)(DT)(DA) (DG) (DC)(DG)(DT)(DT)(DA)(DT) ...String: (DA)(DT)(DC)(DT)(DA)(DC)(DA)(DC)(DG)(DA) (DC)(DG)(DC)(DT)(DC)(DT)(DT)(DC)(DC)(DG) (DA)(DT)(DC)(DT)(DA)(DA)(DT)(DT)(DT) (DA)(DT)(DG)(DT)(DT)(DT)(DG)(DT)(DT)(DA) (DG) (DC)(DG)(DT)(DT)(DA)(DT)(DA)(DC) (DT)(DA)(DT)(DT)(DC)(DT)(DA)(DA)(DT)(DT) (DC)(DT) (DT)(DT)(DG)(DT)(DT)(DT)(DC) (DG)(DG)(DT)(DG)(DG)(DT)(DA)(DT)(DT)(DG) (DT)(DT)(DT) (DA)(DT)(DT)(DT)(DT)(DG) (DT)(DT)(DC)(DC)(DT)(DT)(DT)(DG)(DT)(DG) (DC)(DG)(DT)(DT) (DC)(DA)(DG)(DC)(DT) (DT)(DA)(DA)(DT)(DG)(DC)(DC)(DT)(DA)(DA) (DC)(DG)(DA)(DC)(DA) (DC)(DT)(DC)(DG) (DG)(DA)(DG)(DA)(DT)(DC)(DG)(DG)(DA)(DA) (DG)(DA)(DG)(DC)(DA)(DC) (DA)(DC)(DG) (DT)(DG)(DA)(DT) |
-Macromolecule #6: DNA (147-MER)
| Macromolecule | Name: DNA (147-MER) / type: dna / ID: 6 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism: synthetic construct (others) |
| Molecular weight | Theoretical: 45.484273 KDa |
| Sequence | String: (DA)(DT)(DC)(DA)(DC)(DG)(DT)(DG)(DT)(DG) (DC)(DT)(DC)(DT)(DT)(DC)(DC)(DG)(DA)(DT) (DC)(DT)(DC)(DC)(DG)(DA)(DG)(DT)(DG) (DT)(DC)(DG)(DT)(DT)(DA)(DG)(DG)(DC)(DA) (DT) (DT)(DA)(DA)(DG)(DC)(DT) ...String: (DA)(DT)(DC)(DA)(DC)(DG)(DT)(DG)(DT)(DG) (DC)(DT)(DC)(DT)(DT)(DC)(DC)(DG)(DA)(DT) (DC)(DT)(DC)(DC)(DG)(DA)(DG)(DT)(DG) (DT)(DC)(DG)(DT)(DT)(DA)(DG)(DG)(DC)(DA) (DT) (DT)(DA)(DA)(DG)(DC)(DT)(DG)(DA) (DA)(DC)(DG)(DC)(DA)(DC)(DA)(DA)(DA)(DG) (DG)(DA) (DA)(DC)(DA)(DA)(DA)(DA)(DT) (DA)(DA)(DA)(DC)(DA)(DA)(DT)(DA)(DC)(DC) (DA)(DC)(DC) (DG)(DA)(DA)(DA)(DC)(DA) (DA)(DA)(DG)(DA)(DA)(DT)(DT)(DA)(DG)(DA) (DA)(DT)(DA)(DG) (DT)(DA)(DT)(DA)(DA) (DC)(DG)(DC)(DT)(DA)(DA)(DC)(DA)(DA)(DA) (DC)(DA)(DT)(DA)(DA) (DA)(DT)(DT)(DA) (DG)(DA)(DT)(DC)(DG)(DG)(DA)(DA)(DG)(DA) (DG)(DC)(DG)(DT)(DC)(DG) (DT)(DG)(DT) (DA)(DG)(DA)(DT) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.15 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
| |||||||||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 120 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa / Details: 0.39 mB, 25 mA | |||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 289 K / Instrument: FEI VITROBOT MARK IV Details: The sample was applied onto glow-discharged Quantifoil holey carbon grids. The grids were blotted from both sides for 5-10 seconds at 16*C in a chamber at 100% humidity and plunge-frozen ...Details: The sample was applied onto glow-discharged Quantifoil holey carbon grids. The grids were blotted from both sides for 5-10 seconds at 16*C in a chamber at 100% humidity and plunge-frozen into liquid ethane using a manual plunger.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 30 eV |
| Details | At least 50% of the data were collected at 25* stage tilt in order to partially compensate for preferred orientation of particles on the grid, and to improve angular distribution. |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Number grids imaged: 1 / Average electron dose: 1.125 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 130000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)