+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 | データベース: EMDB / ID: EMD-0452 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
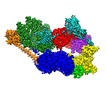
| タイトル | Cryo-EM structure of the human TFIIH core complex | |||||||||||||||
 マップデータ マップデータ | Sharpened and low-pass filtered cryo-EM map. | |||||||||||||||
 試料 試料 |
| |||||||||||||||
 キーワード キーワード | transcription / DNA repair / helicase / multiprotein complex | |||||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報MMXD complex / core TFIIH complex portion of holo TFIIH complex / Cytosolic iron-sulfur cluster assembly / central nervous system myelin formation / positive regulation of mitotic recombination / hair cell differentiation / hair follicle maturation / nucleotide-excision repair factor 3 complex / nucleotide-excision repair, preincision complex assembly / ventricular system development ...MMXD complex / core TFIIH complex portion of holo TFIIH complex / Cytosolic iron-sulfur cluster assembly / central nervous system myelin formation / positive regulation of mitotic recombination / hair cell differentiation / hair follicle maturation / nucleotide-excision repair factor 3 complex / nucleotide-excision repair, preincision complex assembly / ventricular system development / CAK-ERCC2 complex / transcription factor TFIIK complex / embryonic cleavage / DNA 5'-3' helicase / UV protection / adult heart development / regulation of cyclin-dependent protein serine/threonine kinase activity / G protein-coupled receptor internalization / transcription factor TFIIH core complex / transcription factor TFIIH holo complex / cyclin-dependent protein serine/threonine kinase activator activity / nuclear thyroid hormone receptor binding / transcription preinitiation complex / RNA Polymerase I Transcription Termination / DNA 3'-5' helicase / regulation of mitotic cell cycle phase transition / RNA polymerase II general transcription initiation factor activity / transcription factor TFIID complex / erythrocyte maturation / 3'-5' DNA helicase activity / hematopoietic stem cell proliferation / spinal cord development / RNA Pol II CTD phosphorylation and interaction with CE during HIV infection / RNA Pol II CTD phosphorylation and interaction with CE / HIV Transcription Initiation / RNA Polymerase II HIV Promoter Escape / Transcription of the HIV genome / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance / Formation of the Early Elongation Complex / Formation of the HIV-1 Early Elongation Complex / bone mineralization / mRNA Capping / intrinsic apoptotic signaling pathway by p53 class mediator / ATPase activator activity / DNA topological change / RNA Polymerase I Transcription Initiation / regulation of G1/S transition of mitotic cell cycle / hematopoietic stem cell differentiation / embryonic organ development / Tat-mediated elongation of the HIV-1 transcript / Formation of HIV-1 elongation complex containing HIV-1 Tat / transcription elongation by RNA polymerase I / Cyclin E associated events during G1/S transition / response to UV / Formation of HIV elongation complex in the absence of HIV Tat / Cyclin A/B1/B2 associated events during G2/M transition / Cyclin A:Cdk2-associated events at S phase entry / cyclin-dependent protein kinase holoenzyme complex / transcription by RNA polymerase I / RNA Polymerase II Transcription Elongation / Formation of RNA Pol II elongation complex / hormone-mediated signaling pathway / transcription-coupled nucleotide-excision repair / extracellular matrix organization / RNA Polymerase II Pre-transcription Events / positive regulation of smooth muscle cell proliferation / DNA helicase activity / insulin-like growth factor receptor signaling pathway / maturation of SSU-rRNA from tricistronic rRNA transcript (SSU-rRNA, 5.8S rRNA, LSU-rRNA) / determination of adult lifespan / post-embryonic development / nucleotide-excision repair / chromosome segregation / TP53 Regulates Transcription of DNA Repair Genes / transcription initiation at RNA polymerase II promoter / transcription elongation by RNA polymerase II / promoter-specific chromatin binding / RNA Polymerase I Promoter Escape / cellular response to gamma radiation / G1/S transition of mitotic cell cycle / response to calcium ion / NoRC negatively regulates rRNA expression / Dual Incision in GG-NER / Transcription-Coupled Nucleotide Excision Repair (TC-NER) / Formation of TC-NER Pre-Incision Complex / multicellular organism growth / spindle / Formation of Incision Complex in GG-NER / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / Cyclin D associated events in G1 / intracellular protein localization / RUNX1 regulates transcription of genes involved in differentiation of HSCs / 4 iron, 4 sulfur cluster binding / response to oxidative stress / double-stranded DNA binding / 5'-3' DNA helicase activity 類似検索 - 分子機能 | |||||||||||||||
| 生物種 |  Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.7 Å | |||||||||||||||
 データ登録者 データ登録者 | Greber BJ / Toso D | |||||||||||||||
| 資金援助 |  米国, 米国,  スイス, 4件 スイス, 4件
| |||||||||||||||
 引用 引用 |  ジャーナル: Elife / 年: 2019 ジャーナル: Elife / 年: 2019タイトル: The complete structure of the human TFIIH core complex. 著者: Basil J Greber / Daniel B Toso / Jie Fang / Eva Nogales /  要旨: Transcription factor IIH (TFIIH) is a heterodecameric protein complex critical for transcription initiation by RNA polymerase II and nucleotide excision DNA repair. The TFIIH core complex is ...Transcription factor IIH (TFIIH) is a heterodecameric protein complex critical for transcription initiation by RNA polymerase II and nucleotide excision DNA repair. The TFIIH core complex is sufficient for its repair functions and harbors the XPB and XPD DNA-dependent ATPase/helicase subunits, which are affected by human disease mutations. Transcription initiation additionally requires the CdK activating kinase subcomplex. Previous structural work has provided only partial insight into the architecture of TFIIH and its interactions within transcription pre-initiation complexes. Here, we present the complete structure of the human TFIIH core complex, determined by phase-plate cryo-electron microscopy at 3.7 Å resolution. The structure uncovers the molecular basis of TFIIH assembly, revealing how the recruitment of XPB by p52 depends on a pseudo-symmetric dimer of homologous domains in these two proteins. The structure also suggests a function for p62 in the regulation of XPD, and allows the mapping of previously unresolved human disease mutations. | |||||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| ムービー |
 ムービービューア ムービービューア |
|---|---|
| 構造ビューア | EMマップ:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| 添付画像 |
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_0452.map.gz emd_0452.map.gz | 59.6 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-0452-v30.xml emd-0452-v30.xml emd-0452.xml emd-0452.xml | 32.1 KB 32.1 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_0452.png emd_0452.png | 166 KB | ||
| Filedesc metadata |  emd-0452.cif.gz emd-0452.cif.gz | 8.9 KB | ||
| その他 |  emd_0452_half_map_1.map.gz emd_0452_half_map_1.map.gz emd_0452_half_map_2.map.gz emd_0452_half_map_2.map.gz | 49.7 MB 49.6 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-0452 http://ftp.pdbj.org/pub/emdb/structures/EMD-0452 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0452 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-0452 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_0452_validation.pdf.gz emd_0452_validation.pdf.gz | 960.1 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_0452_full_validation.pdf.gz emd_0452_full_validation.pdf.gz | 959.7 KB | 表示 | |
| XML形式データ |  emd_0452_validation.xml.gz emd_0452_validation.xml.gz | 11.8 KB | 表示 | |
| CIF形式データ |  emd_0452_validation.cif.gz emd_0452_validation.cif.gz | 13.9 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0452 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0452 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0452 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-0452 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  6nmiMC  0587C  0588C  0589C  0602C  0603C  0604C M: このマップから作成された原子モデル C: 同じ文献を引用 ( |
|---|---|
| 類似構造データ | |
| 電子顕微鏡画像生データ |  EMPIAR-10430 (タイトル: Phase-plate cryo-EM of human TFIIH / Data size: 9.2 TB EMPIAR-10430 (タイトル: Phase-plate cryo-EM of human TFIIH / Data size: 9.2 TBData #1: Unaligned movies of human TFIIH, using the Volta phase plate, dataset 1 [micrographs - multiframe] Data #2: Unaligned movies of human TFIIH, using the Volta phase plate, dataset 2 [micrographs - multiframe] Data #3: Unaligned movies of human TFIIH, using the Volta phase plate, dataset 3 [micrographs - multiframe] Data #4: Unaligned movies of human TFIIH, using the Volta phase plate, dataset 4 [micrographs - multiframe] Data #5: Unaligned movies of human TFIIH, using the Volta phase plate, dataset 5 [micrographs - multiframe] Data #6: Unaligned movies of human TFIIH, using the Volta phase plate, dataset 6 [micrographs - multiframe]) |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_0452.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_0452.map.gz / 形式: CCP4 / 大きさ: 64 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
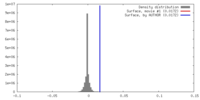
| 注釈 | Sharpened and low-pass filtered cryo-EM map. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 1.15 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
CCP4マップ ヘッダ情報:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-添付データ
-ハーフマップ: Unfiltered half-map.
| ファイル | emd_0452_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
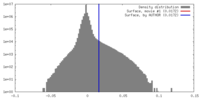
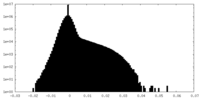
| 注釈 | Unfiltered half-map. | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: Unfiltered half-map.
| ファイル | emd_0452_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
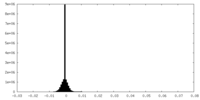
| 注釈 | Unfiltered half-map. | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
+全体 : Transcription factor IIH (TFIIH)
+超分子 #1: Transcription factor IIH (TFIIH)
+分子 #1: General transcription and DNA repair factor IIH helicase subunit XPB
+分子 #2: General transcription and DNA repair factor IIH helicase subunit XPD
+分子 #3: General transcription factor IIH subunit 1, p62
+分子 #4: General transcription factor IIH subunit 4, p52
+分子 #5: General transcription factor IIH subunit 2, p44
+分子 #6: General transcription factor IIH subunit 3, p34
+分子 #7: General transcription factor IIH subunit 5, p8
+分子 #8: CDK-activating kinase assembly factor MAT1
+分子 #9: IRON/SULFUR CLUSTER
+分子 #10: ZINC ION
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 濃度 | 0.0049 mg/mL | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 緩衝液 | pH: 7.9 構成要素:
| ||||||||||||||
| グリッド | モデル: C-flat / 材質: COPPER / メッシュ: 400 / 支持フィルム - #0 - Film type ID: 1 / 支持フィルム - #0 - 材質: CARBON / 支持フィルム - #0 - トポロジー: HOLEY / 支持フィルム - #1 - Film type ID: 2 / 支持フィルム - #1 - 材質: CARBON / 支持フィルム - #1 - トポロジー: CONTINUOUS / 前処理 - タイプ: PLASMA CLEANING / 詳細: Gatan Solarus | ||||||||||||||
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % / チャンバー内温度: 277 K / 装置: FEI VITROBOT MARK IV 詳細: A thin film of continuous carbon was floated onto Protochips C-flat CF-4/2 holey carbon grids and glow discharged or plasma cleaned before application of 4 uL of sample solution.. |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 特殊光学系 | 位相板: VOLTA PHASE PLATE / エネルギーフィルター - 名称: GIF Quantum LS / エネルギーフィルター - スリット幅: 20 eV |
| 詳細 | Residual beam tilt corrected in RELION 3. |
| 撮影 | フィルム・検出器のモデル: GATAN K2 SUMMIT (4k x 4k) 検出モード: SUPER-RESOLUTION / 撮影したグリッド数: 7 / 実像数: 21437 / 平均露光時間: 8.25 sec. / 平均電子線量: 50.0 e/Å2 詳細: Images were collected as dose-fractionated movie frames (33 or 50 frames per exposure). |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 倍率(補正後): 43478 / 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / Cs: 2.7 mm 最大 デフォーカス(公称値): 0.7000000000000001 µm 最小 デフォーカス(公称値): 0.5 µm |
| 試料ステージ | 試料ホルダーモデル: FEI TITAN KRIOS AUTOGRID HOLDER ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
+ 画像解析
画像解析
-原子モデル構築 1
| 初期モデル |
| ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 詳細 | Reference structures and homology models were docked and subsequently completely rebuilt according to the density. Parts of the structure were traced de novo. | ||||||||||||||||
| 精密化 | 空間: REAL / プロトコル: RIGID BODY FIT | ||||||||||||||||
| 得られたモデル |  PDB-6nmi: |
 ムービー
ムービー コントローラー
コントローラー





























 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)