+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6xd3 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Structure of the human CAK in complex with THZ1 | |||||||||||||||
 Components Components |
| |||||||||||||||
 Keywords Keywords | TRANSFERASE / Kinase / transcription / cell cycle / complex | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationventricular system development / snRNA transcription by RNA polymerase II / CAK-ERCC2 complex / transcription factor TFIIK complex / adult heart development / transcription factor TFIIH holo complex / transcription factor TFIIH core complex / cyclin-dependent protein serine/threonine kinase activator activity / [RNA-polymerase]-subunit kinase / RNA Polymerase I Transcription Termination ...ventricular system development / snRNA transcription by RNA polymerase II / CAK-ERCC2 complex / transcription factor TFIIK complex / adult heart development / transcription factor TFIIH holo complex / transcription factor TFIIH core complex / cyclin-dependent protein serine/threonine kinase activator activity / [RNA-polymerase]-subunit kinase / RNA Polymerase I Transcription Termination / cyclin-dependent protein serine/threonine kinase regulator activity / RNA Pol II CTD phosphorylation and interaction with CE during HIV infection / RNA Pol II CTD phosphorylation and interaction with CE / Formation of the Early Elongation Complex / Formation of the HIV-1 Early Elongation Complex / mRNA Capping / HIV Transcription Initiation / RNA Polymerase II HIV Promoter Escape / Transcription of the HIV genome / RNA Polymerase II Promoter Escape / RNA Polymerase II Transcription Pre-Initiation And Promoter Opening / RNA Polymerase II Transcription Initiation / RNA Polymerase II Transcription Initiation And Promoter Clearance / regulation of G1/S transition of mitotic cell cycle / RNA Polymerase I Transcription Initiation / RNA polymerase II transcribes snRNA genes / Tat-mediated elongation of the HIV-1 transcript / Formation of HIV-1 elongation complex containing HIV-1 Tat / cyclin-dependent kinase / Formation of HIV elongation complex in the absence of HIV Tat / cyclin-dependent protein serine/threonine kinase activity / ATP-dependent activity, acting on DNA / Cyclin E associated events during G1/S transition / RNA Polymerase II Transcription Elongation / Cyclin A/B1/B2 associated events during G2/M transition / cyclin-dependent protein kinase holoenzyme complex / Formation of RNA Pol II elongation complex / Cyclin A:Cdk2-associated events at S phase entry / RNA Polymerase II Pre-transcription Events / RNA polymerase II CTD heptapeptide repeat kinase activity / male germ cell nucleus / TP53 Regulates Transcription of DNA Repair Genes / nucleotide-excision repair / transcription initiation at RNA polymerase II promoter / RNA Polymerase I Promoter Escape / positive regulation of smooth muscle cell proliferation / NoRC negatively regulates rRNA expression / Transcription-Coupled Nucleotide Excision Repair (TC-NER) / Formation of TC-NER Pre-Incision Complex / fibrillar center / Formation of Incision Complex in GG-NER / response to calcium ion / Dual incision in TC-NER / Gap-filling DNA repair synthesis and ligation in TC-NER / G1/S transition of mitotic cell cycle / Cyclin D associated events in G1 / RUNX1 regulates transcription of genes involved in differentiation of HSCs / transcription by RNA polymerase II / regulation of cell cycle / protein stabilization / protein kinase activity / cell division / protein serine kinase activity / DNA repair / protein serine/threonine kinase activity / negative regulation of apoptotic process / regulation of transcription by RNA polymerase II / perinuclear region of cytoplasm / positive regulation of transcription by RNA polymerase II / zinc ion binding / nucleoplasm / ATP binding / nucleus / plasma membrane / cytosol / cytoplasm Similarity search - Function | |||||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||||||||
 Authors Authors | Greber, B.J. / Perez-Bertoldi, J.M. / Lim, K. / Iavarone, A.T. / Toso, D.B. / Nogales, E. | |||||||||||||||
| Funding support |  United States, 4items United States, 4items
| |||||||||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2020 Journal: Proc Natl Acad Sci U S A / Year: 2020Title: The cryoelectron microscopy structure of the human CDK-activating kinase. Authors: Basil J Greber / Juan M Perez-Bertoldi / Kif Lim / Anthony T Iavarone / Daniel B Toso / Eva Nogales /  Abstract: The human CDK-activating kinase (CAK), a complex composed of cyclin-dependent kinase (CDK) 7, cyclin H, and MAT1, is a critical regulator of transcription initiation and the cell cycle. It acts by ...The human CDK-activating kinase (CAK), a complex composed of cyclin-dependent kinase (CDK) 7, cyclin H, and MAT1, is a critical regulator of transcription initiation and the cell cycle. It acts by phosphorylating the C-terminal heptapeptide repeat domain of the RNA polymerase II (Pol II) subunit RPB1, which is an important regulatory event in transcription initiation by Pol II, and it phosphorylates the regulatory T-loop of CDKs that control cell cycle progression. Here, we have determined the three-dimensional (3D) structure of the catalytic module of human CAK, revealing the structural basis of its assembly and providing insight into CDK7 activation in this context. The unique third component of the complex, MAT1, substantially extends the interaction interface between CDK7 and cyclin H, explaining its role as a CAK assembly factor, and it forms interactions with the CDK7 T-loop, which may contribute to enhancing CAK activity. We have also determined the structure of the CAK in complex with the covalently bound inhibitor THZ1 in order to provide insight into the binding of inhibitors at the CDK7 active site and to aid in the rational design of therapeutic compounds. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6xd3.cif.gz 6xd3.cif.gz | 133.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6xd3.ent.gz pdb6xd3.ent.gz | 97.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6xd3.json.gz 6xd3.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Summary document |  6xd3_validation.pdf.gz 6xd3_validation.pdf.gz | 1.4 MB | Display |  wwPDB validaton report wwPDB validaton report |
|---|---|---|---|---|
| Full document |  6xd3_full_validation.pdf.gz 6xd3_full_validation.pdf.gz | 1.4 MB | Display | |
| Data in XML |  6xd3_validation.xml.gz 6xd3_validation.xml.gz | 30.2 KB | Display | |
| Data in CIF |  6xd3_validation.cif.gz 6xd3_validation.cif.gz | 42.3 KB | Display | |
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/xd/6xd3 https://data.pdbj.org/pub/pdb/validation_reports/xd/6xd3 ftp://data.pdbj.org/pub/pdb/validation_reports/xd/6xd3 ftp://data.pdbj.org/pub/pdb/validation_reports/xd/6xd3 | HTTPS FTP |
-Related structure data
| Related structure data |  22131MC  6xbzC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | |
| EM raw data |  EMPIAR-10438 (Title: Single-particle cryo-EM of the human CDK-activating kinase in complex with THZ1 EMPIAR-10438 (Title: Single-particle cryo-EM of the human CDK-activating kinase in complex with THZ1Data size: 2.4 TB Data #1: Unaligned movies of human CAK-THZ1 [micrographs - multiframe]) |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 10234.531 Da / Num. of mol.: 1 / Fragment: residues 220-309 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: MNAT1, CAP35, MAT1, RNF66 / Cell line (production host): High5 / Production host: Homo sapiens (human) / Gene: MNAT1, CAP35, MAT1, RNF66 / Cell line (production host): High5 / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: P51948 Trichoplusia ni (cabbage looper) / References: UniProt: P51948 |
|---|---|
| #2: Protein | Mass: 37695.473 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CCNH / Production host: Homo sapiens (human) / Gene: CCNH / Production host:  Trichoplusia ni (cabbage looper) / References: UniProt: P51946 Trichoplusia ni (cabbage looper) / References: UniProt: P51946 |
| #3: Protein | Mass: 39442.574 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CDK7, CAK, CAK1, CDKN7, MO15, STK1 / Production host: Homo sapiens (human) / Gene: CDK7, CAK, CAK1, CDKN7, MO15, STK1 / Production host:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper)References: UniProt: P50613, cyclin-dependent kinase, [RNA-polymerase]-subunit kinase |
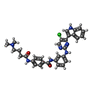
| #4: Chemical | ChemComp-V0G / |
| Has ligand of interest | Y |
| Has protein modification | Y |
| Nonpolymer details | The authors state that the protein is covalently modified at CDK7 cysteine 312 with a ligand. The ...The authors state that the protein is covalently modified at CDK7 cysteine 312 with a ligand. The ligand in the entry is THZ1-R, which is the form that is present after adduct formation, during which one C=C double bond in THZ1 gets reduced by Michael addition. |
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: CDK-activating kinase assembly factor MAT1, Cyclin-H, Cyclin-dependent kinase 7 (E.C.2.7.11.22,2.7.11.23) Type: COMPLEX Details: Recombinantly expressed as a trimeric complex in insect cells (MAT1 subunit truncated) and then modified with covalent inhibitor THZ1 Entity ID: #1-#3 / Source: RECOMBINANT | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.08 MDa / Experimental value: NO | ||||||||||||||||||||||||||||||
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) | ||||||||||||||||||||||||||||||
| Source (recombinant) | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) | ||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.9 | ||||||||||||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||||||||||||
| Specimen | Conc.: 0.2 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES | ||||||||||||||||||||||||||||||
| Specimen support | Grid material: GOLD / Grid type: UltrAuFoil R1.2/1.3 | ||||||||||||||||||||||||||||||
| Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 278 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TALOS ARCTICA |
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD / Calibrated magnification: 72886 X / Nominal defocus max: 2500 nm / Nominal defocus min: 300 nm / Cs: 2.7 mm / C2 aperture diameter: 50 µm / Alignment procedure: ZEMLIN TABLEAU |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 2 sec. / Electron dose: 69 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) / Num. of grids imaged: 1 / Num. of real images: 5007 / Details: 69 frames per movie |
- Processing
Processing
| Software | Name: PHENIX / Version: 1.18.2_3874: / Classification: refinement | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||
| CTF correction | Details: CTF estimation with CTFFIND4, CTF correction in RELION 3.1 during reconstruction. Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 4884787 / Details: 3D-template picking | ||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||||||||||
| 3D reconstruction | Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 31198 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: OTHER / Space: REAL | ||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | PDB-ID: 6XBZ Accession code: 6XBZ / Source name: PDB / Type: experimental model | ||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj