[English] 日本語
 Yorodumi
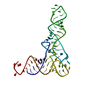
Yorodumi- PDB-4v71: E. coli 70S-fMetVal-tRNAVal-tRNAfMet complex in intermediate pre-... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 4v71 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | E. coli 70S-fMetVal-tRNAVal-tRNAfMet complex in intermediate pre-translocation state (pre2) | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords | RIBOSOME / cryo-EM refinement / tRNA / translocation intermediate | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationstringent response / transcription antitermination factor activity, RNA binding / ornithine decarboxylase inhibitor activity / misfolded RNA binding / Group I intron splicing / RNA folding / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity ...stringent response / transcription antitermination factor activity, RNA binding / ornithine decarboxylase inhibitor activity / misfolded RNA binding / Group I intron splicing / RNA folding / transcriptional attenuation / endoribonuclease inhibitor activity / positive regulation of ribosome biogenesis / RNA-binding transcription regulator activity / translational termination / negative regulation of cytoplasmic translation / four-way junction DNA binding / DnaA-L2 complex / translation repressor activity / negative regulation of translational initiation / regulation of mRNA stability / negative regulation of DNA-templated DNA replication initiation / mRNA regulatory element binding translation repressor activity / positive regulation of RNA splicing / assembly of large subunit precursor of preribosome / cytosolic ribosome assembly / response to reactive oxygen species / regulation of DNA-templated transcription elongation / ribosome assembly / transcription elongation factor complex / transcription antitermination / DNA endonuclease activity / regulation of cell growth / DNA-templated transcription termination / response to radiation / maintenance of translational fidelity / mRNA 5'-UTR binding / regulation of translation / large ribosomal subunit / ribosome biogenesis / transferase activity / ribosome binding / ribosomal small subunit biogenesis / ribosomal small subunit assembly / ribosomal large subunit assembly / 5S rRNA binding / small ribosomal subunit / small ribosomal subunit rRNA binding / cytosolic small ribosomal subunit / large ribosomal subunit rRNA binding / cytosolic large ribosomal subunit / cytoplasmic translation / tRNA binding / negative regulation of translation / rRNA binding / structural constituent of ribosome / ribosome / translation / hydrolase activity / response to antibiotic / negative regulation of DNA-templated transcription / mRNA binding / DNA binding / RNA binding / zinc ion binding / membrane / cytoplasm / cytosol Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | ELECTRON MICROSCOPY / single particle reconstruction / cryo EM / Resolution: 20 Å | ||||||||||||
 Authors Authors | Blau, C. / Bock, L.V. / Schroder, G.F. / Davydov, I. / Fischer, N. / Stark, H. / Rodnina, M.V. / Vaiana, A.C. / Grubmuller, H. | ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2013 Journal: Nat Struct Mol Biol / Year: 2013Title: Energy barriers and driving forces in tRNA translocation through the ribosome. Authors: Lars V Bock / Christian Blau / Gunnar F Schröder / Iakov I Davydov / Niels Fischer / Holger Stark / Marina V Rodnina / Andrea C Vaiana / Helmut Grubmüller /  Abstract: During protein synthesis, tRNAs move from the ribosome's aminoacyl to peptidyl to exit sites. Here we investigate conformational motions during spontaneous translocation, using molecular dynamics ...During protein synthesis, tRNAs move from the ribosome's aminoacyl to peptidyl to exit sites. Here we investigate conformational motions during spontaneous translocation, using molecular dynamics simulations of 13 intermediate-translocation-state models obtained by combining Escherichia coli ribosome crystal structures with cryo-EM data. Resolving fast transitions between states, we find that tRNA motions govern the transition rates within the pre- and post-translocation states. Intersubunit rotations and L1-stalk motion exhibit fast intrinsic submicrosecond dynamics. The L1 stalk drives the tRNA from the peptidyl site and links intersubunit rotation to translocation. Displacement of tRNAs is controlled by 'sliding' and 'stepping' mechanisms involving conserved L16, L5 and L1 residues, thus ensuring binding to the ribosome despite large-scale tRNA movement. Our results complement structural data with a time axis, intrinsic transition rates and molecular forces, revealing correlated functional motions inaccessible by other means. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  4v71.cif.gz 4v71.cif.gz | 3.3 MB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb4v71.ent.gz pdb4v71.ent.gz | Display |  PDB format PDB format | |
| PDBx/mmJSON format |  4v71.json.gz 4v71.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/v7/4v71 https://data.pdbj.org/pub/pdb/validation_reports/v7/4v71 ftp://data.pdbj.org/pub/pdb/validation_reports/v7/4v71 ftp://data.pdbj.org/pub/pdb/validation_reports/v7/4v71 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  1717M  2472C  2473C  2474C  2475C  4v6yC  4v6zC  4v70C  4v72C  4v73C  4v74C  4v75C  4v76C  4v77C  4v78C  4v79C  4v7aC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-30S ribosomal protein ... , 20 types, 20 molecules ABACADAEAFAGAHAIAJAKALAMANAOAPAQARASATAU
| #1: Protein | Mass: 24277.992 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #2: Protein | Mass: 23207.990 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #3: Protein | Mass: 23514.199 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #4: Protein | Mass: 15828.328 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #5: Protein | Mass: 11667.380 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #6: Protein | Mass: 16788.453 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #7: Protein | Mass: 14146.557 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #8: Protein | Mass: 14580.919 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #9: Protein | Mass: 11221.034 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #10: Protein | Mass: 12513.237 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #11: Protein | Mass: 13768.157 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #12: Protein | Mass: 12657.843 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #13: Protein | Mass: 11606.560 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #14: Protein | Mass: 10290.816 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #15: Protein | Mass: 9063.426 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #16: Protein | Mass: 9287.992 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #17: Protein | Mass: 6490.523 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #18: Protein | Mass: 9081.672 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #19: Protein | Mass: 9532.228 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #20: Protein | Mass: 6091.125 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
-RNA chain , 6 types, 6 molecules AAA1A2A3BABB
| #21: RNA chain | Mass: 496892.375 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
|---|---|
| #22: RNA chain | Mass: 24617.768 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #23: RNA chain | Mass: 4693.746 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: mRNA |
| #24: RNA chain | Mass: 24848.918 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #54: RNA chain | Mass: 941306.188 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
| #55: RNA chain | Mass: 38177.762 Da / Num. of mol.: 1 / Source method: isolated from a natural source / Source: (natural)  |
+50S ribosomal protein ... , 30 types, 30 molecules BCBDBEBFBGBHBIBJBKBLBMBNBOBPBQBRBSBTBUBVBWBXBYBZB0B1B2B3B4B5
-Non-polymers , 2 types, 2 molecules 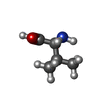


| #57: Chemical | ChemComp-VAL / |
|---|---|
| #58: Chemical | ChemComp-FME / |
-Details
| Has protein modification | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method: ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method: single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: E. coli 70S-fMetVal-tRNAVal-tRNAfMet complex / Type: RIBOSOME |
|---|---|
| Buffer solution | Name: 50 mM Tris-HCl, 70 mM NH4Cl, 30 mM KCl, 7 mM MgCl2, 0.6 mM spermine, 0.4 mM spermidine pH: 7.5 Details: 50 mM Tris-HCl, 70 mM NH4Cl, 30 mM KCl, 7 mM MgCl2, 0.6 mM spermine, 0.4 mM spermidine |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied: NO / Vitrification applied: YES |
| Vitrification | Instrument: HOMEMADE PLUNGER / Cryogen name: ETHANE / Humidity: 75 % Details: Manual blotting for about 2 seconds prior to plunging into liquid ethane (custom-built CEVS vitrification instrument with dew point temperature adjusted to 18 degrees C) Method: Manual blotting for about 2 seconds Time resolved state: Samples were vitrified at different time points along the reaction coordinate (1, 2, 5 and 20 minutes after addition of deacylated tRNAfMet to 70S-fMetVal-tRNAVal complexes). |
- Electron microscopy imaging
Electron microscopy imaging
| Microscopy | Model: FEI/PHILIPS CM200FEG / Date: May 11, 2008 Details: Objective lens astigmatism was corrected at 200,000 times magnification |
|---|---|
| Electron gun | Electron source:  FIELD EMISSION GUN / Accelerating voltage: 160 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 160 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: BRIGHT FIELD / Nominal magnification: 161000 X / Calibrated magnification: 162740 X / Nominal defocus max: 2000 nm / Nominal defocus min: 500 nm / Cs: 2 mm |
| Specimen holder | Specimen holder model: GATAN LIQUID NITROGEN / Specimen holder type: Eucentric / Temperature: 77 K |
| Image recording | Electron dose: 20 e/Å2 / Film or detector model: GENERIC TVIPS (4k x 4k) / Details: 4k CCD camera (TVIPS) |
- Processing
Processing
| EM software |
| |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CTF correction | Details: local | |||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry: C1 (asymmetric) | |||||||||||||||||||||||||||||||||||
| 3D reconstruction | Method: Projection matching / Resolution: 20 Å / Resolution method: FSC 0.5 CUT-OFF / Num. of particles: 1904 / Nominal pixel size: 2.8 Å / Actual pixel size: 2.8 Å Details: Final maps were calculated from 13 datasets acquired at different time points, computationally sorted into distinct substates. Symmetry type: POINT | |||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL / Target criteria: Cross-correlation coefficient Details: METHOD--Cross-correlation gradient based REFINEMENT PROTOCOL--flexible fitting, dynamic elastic network | |||||||||||||||||||||||||||||||||||
| Atomic model building |
|
 Movie
Movie Controller
Controller






























 PDBj
PDBj