| Entry | Database: PDB / ID: 6uap
|
|---|
| Title | Crystal structure of tryptophan synthase from M. tuberculosis - open form with BRD6309 bound |
|---|
 Components Components | (Tryptophan synthase ...) x 2 |
|---|
 Keywords Keywords | Lyase/Lyase Inhibitor / PLP / heterotetramer / amino acid biosynthesis / substrate channeling / allostery / Structural Genomics / Center for Structural Genomics of Infectious Diseases / CSGID / Lyase-Lyase Inhibitor complex |
|---|
| Function / homology |  Function and homology information Function and homology information
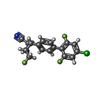
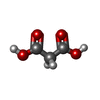
tryptophan synthase / tryptophan synthase activity / L-tryptophan biosynthetic process / plasma membrane / cytoplasm / cytosolSimilarity search - Function Tryptophan synthase, alpha chain / Tryptophan synthase, alpha chain, active site / Tryptophan synthase alpha chain / Tryptophan synthase alpha chain signature. / Tryptophan synthase, beta chain, conserved site / Tryptophan synthase, beta chain / Tryptophan synthase beta chain/beta chain-like / Tryptophan synthase beta chain pyridoxal-phosphate attachment site. / Rossmann fold - #1100 / Pyridoxal-phosphate dependent enzyme ...Tryptophan synthase, alpha chain / Tryptophan synthase, alpha chain, active site / Tryptophan synthase alpha chain / Tryptophan synthase alpha chain signature. / Tryptophan synthase, beta chain, conserved site / Tryptophan synthase, beta chain / Tryptophan synthase beta chain/beta chain-like / Tryptophan synthase beta chain pyridoxal-phosphate attachment site. / Rossmann fold - #1100 / Pyridoxal-phosphate dependent enzyme / Pyridoxal-phosphate dependent enzyme / Tryptophan synthase beta subunit-like PLP-dependent enzyme / Ribulose-phosphate binding barrel / Aldolase class I / Aldolase-type TIM barrel / TIM Barrel / Alpha-Beta Barrel / Rossmann fold / 3-Layer(aba) Sandwich / Alpha BetaSimilarity search - Domain/homology ACETATE ION / FORMIC ACID / Chem-H9V / MALONIC ACID / D-MALATE / Tryptophan synthase beta chain / Tryptophan synthase alpha chainSimilarity search - Component |
|---|
| Biological species |   Mycobacterium tuberculosis (bacteria) Mycobacterium tuberculosis (bacteria) |
|---|
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.745 Å MOLECULAR REPLACEMENT / Resolution: 2.745 Å |
|---|
 Authors Authors | Chang, C. / Michalska, K. / Maltseva, N.I. / Jedrzejczak, R. / McCarren, P. / Nag, P.P. / Joachimiak, A. / Satchell, K. / Center for Structural Genomics of Infectious Diseases (CSGID) |
|---|
| Funding support |  Canada, 1items Canada, 1items | Organization | Grant number | Country |
|---|
| Structure-guided Drug Discovery Coalition | |  Canada Canada |
|
|---|
 Citation Citation |  Journal: To be Published Journal: To be Published
Title: Crystal structure of tryptophan synthase from M. tuberculosis - open form with BRD6309 bound
Authors: Chang, C. / Michalska, K. / Maltseva, N.I. / Jedrzejczak, R. / McCarren, P. / Nag, P.P. / Joachimiak, A. / Satchell, K. |
|---|
| History | | Deposition | Sep 11, 2019 | Deposition site: RCSB / Processing site: RCSB |
|---|
| Supersession | Oct 30, 2019 | ID: 6DU1 |
|---|
| Revision 1.0 | Oct 30, 2019 | Provider: repository / Type: Initial release |
|---|
| Revision 1.1 | Apr 2, 2025 | Group: Advisory / Data collection ...Advisory / Data collection / Database references / Structure summary
Category: chem_comp_atom / chem_comp_bond ...chem_comp_atom / chem_comp_bond / database_2 / pdbx_entry_details / pdbx_modification_feature / pdbx_unobs_or_zero_occ_atoms
Item: _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_entry_details.has_protein_modification |
|---|
|
|---|
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.745 Å
MOLECULAR REPLACEMENT / Resolution: 2.745 Å  Authors
Authors Canada, 1items
Canada, 1items  Citation
Citation Journal: To be Published
Journal: To be Published Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 6uap.cif.gz
6uap.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb6uap.ent.gz
pdb6uap.ent.gz PDB format
PDB format 6uap.json.gz
6uap.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/ua/6uap
https://data.pdbj.org/pub/pdb/validation_reports/ua/6uap ftp://data.pdbj.org/pub/pdb/validation_reports/ua/6uap
ftp://data.pdbj.org/pub/pdb/validation_reports/ua/6uap Links
Links Assembly
Assembly


 Components
Components Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria)
Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria)
 Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria)
Mycobacterium tuberculosis (strain ATCC 25618 / H37Rv) (bacteria)











 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  APS
APS  / Beamline: 19-ID / Wavelength: 0.9788 Å
/ Beamline: 19-ID / Wavelength: 0.9788 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 2.745→29.651 Å / SU ML: 0.35 / Cross valid method: THROUGHOUT / σ(F): 1.34 / Phase error: 22.28
MOLECULAR REPLACEMENT / Resolution: 2.745→29.651 Å / SU ML: 0.35 / Cross valid method: THROUGHOUT / σ(F): 1.34 / Phase error: 22.28  Movie
Movie Controller
Controller






















 PDBj
PDBj








