[English] 日本語
 Yorodumi
Yorodumi- PDB-6jen: Structure of Phytolacca americana UGT2 complexed with UDP-2fluoro... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6jen | ||||||
|---|---|---|---|---|---|---|---|
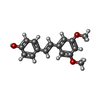
| Title | Structure of Phytolacca americana UGT2 complexed with UDP-2fluoro-glucose and pterostilbene | ||||||
 Components Components | Glycosyltransferase | ||||||
 Keywords Keywords | TRANSFERASE / glycosyltransferase | ||||||
| Function / homology |  Function and homology information Function and homology informationUDP-glycosyltransferase activity / Transferases; Glycosyltransferases; Hexosyltransferases Similarity search - Function | ||||||
| Biological species |  Phytolacca americana (American pokeweed) Phytolacca americana (American pokeweed) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.65 Å MOLECULAR REPLACEMENT / Resolution: 2.65 Å | ||||||
 Authors Authors | Maharjan, R. / Fukuda, Y. / Nakayama, T. / Hamada, H. / Ozaki, S. / Inoue, T. | ||||||
| Funding support |  Japan, 1items Japan, 1items
| ||||||
 Citation Citation |  Journal: Biochemistry / Year: 2020 Journal: Biochemistry / Year: 2020Title: An Ambidextrous Polyphenol GlycosyltransferasePaGT2 fromPhytolacca americana. Authors: Maharjan, R. / Fukuda, Y. / Shimomura, N. / Nakayama, T. / Okimoto, Y. / Kawakami, K. / Nakayama, T. / Hamada, H. / Inoue, T. / Ozaki, S.I. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6jen.cif.gz 6jen.cif.gz | 266.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6jen.ent.gz pdb6jen.ent.gz | 212.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6jen.json.gz 6jen.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/je/6jen https://data.pdbj.org/pub/pdb/validation_reports/je/6jen ftp://data.pdbj.org/pub/pdb/validation_reports/je/6jen ftp://data.pdbj.org/pub/pdb/validation_reports/je/6jen | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6jelC  6jemC  2vchS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 | 
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: _ / Beg auth comp-ID: ALA / Beg label comp-ID: ALA / Refine code: _
NCS ensembles :
|
- Components
Components
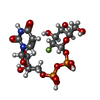
| #1: Protein | Mass: 53829.195 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Phytolacca americana (American pokeweed) Phytolacca americana (American pokeweed)Gene: PaGT2 / Plasmid: pCold / Production host:  References: UniProt: B5MGN7, Transferases; Glycosyltransferases; Hexosyltransferases #2: Chemical | #3: Chemical | Has ligand of interest | N | Sequence details | The sequence database with ID B5MGN7 contains residue TYR at position 211. However, after observing ...The sequence database with ID B5MGN7 contains residue TYR at position 211. However, after observing the discrepancy, authors confirmed that residue at 211 is ASN and not TYR. The depositors will soon correct the sequence in the database. | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.47 Å3/Da / Density % sol: 50.11 % |
|---|---|
| Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 8 Details: 0.11 M potassium citrate 0.06 M lithium citrate 0.11 M sodium phosphate 23-25% w/v PEG 6000 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SPring-8 SPring-8  / Beamline: BL44XU / Wavelength: 0.9 Å / Beamline: BL44XU / Wavelength: 0.9 Å |
| Detector | Type: RAYONIX MX300HE / Detector: CCD / Date: May 27, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9 Å / Relative weight: 1 |
| Reflection | Resolution: 2.65→50 Å / Num. obs: 47340 / % possible obs: 99.8 % / Redundancy: 6.6 % / Rmerge(I) obs: 0.071 / Rrim(I) all: 0.077 / Net I/σ(I): 15.7 |
| Reflection shell | Resolution: 2.65→2.74 Å / Mean I/σ(I) obs: 2 / Num. unique obs: 4530 / CC1/2: 0.694 / % possible all: 98.6 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2VCH Resolution: 2.65→49.68 Å / Cor.coef. Fo:Fc: 0.959 / Cor.coef. Fo:Fc free: 0.94 / Cross valid method: THROUGHOUT / ESU R: 0.848 / ESU R Free: 0.319 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 85 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 2.65→49.68 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller












 PDBj
PDBj