Entry Database : PDB / ID : 6bplTitle E. coli MsbA in complex with LPS and inhibitor G907 Lipid A export ATP-binding/permease protein MsbA Keywords / / / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Escherichia coli O6:H1 (bacteria)Method / / / Resolution : 2.908 Å Authors Ho, H. / Koth, C.M. / Payandeh, J. Journal : Nature / Year : 2018Title : Structural basis for dual-mode inhibition of the ABC transporter MsbA.Authors: Ho, H. / Miu, A. / Alexander, M.K. / Garcia, N.K. / Oh, A. / Zilberleyb, I. / Reichelt, M. / Austin, C.D. / Tam, C. / Shriver, S. / Hu, H. / Labadie, S.S. / Liang, J. / Wang, L. / Wang, J. / ... Authors : Ho, H. / Miu, A. / Alexander, M.K. / Garcia, N.K. / Oh, A. / Zilberleyb, I. / Reichelt, M. / Austin, C.D. / Tam, C. / Shriver, S. / Hu, H. / Labadie, S.S. / Liang, J. / Wang, L. / Wang, J. / Lu, Y. / Purkey, H.E. / Quinn, J. / Franke, Y. / Clark, K. / Beresini, M.H. / Tan, M.W. / Sellers, B.D. / Maurer, T. / Koehler, M.F.T. / Wecksler, A.T. / Kiefer, J.R. / Verma, V. / Xu, Y. / Nishiyama, M. / Payandeh, J. / Koth, C.M. History Deposition Nov 23, 2017 Deposition site / Processing site Revision 1.0 May 2, 2018 Provider / Type Revision 1.1 May 16, 2018 Group / Database references / Category / citation_authorItem / _citation.title / _citation_author.nameRevision 1.2 May 30, 2018 Group / Database references / Category Item / _citation.page_first / _citation.page_lastRevision 1.3 Sep 12, 2018 Group / Database references / Source and taxonomyCategory entity_src_gen / struct_ref ... entity_src_gen / struct_ref / struct_ref_seq / struct_ref_seq_dif Item _entity_src_gen.gene_src_strain / _entity_src_gen.pdbx_gene_src_gene ... _entity_src_gen.gene_src_strain / _entity_src_gen.pdbx_gene_src_gene / _entity_src_gen.pdbx_gene_src_ncbi_taxonomy_id / _entity_src_gen.pdbx_gene_src_scientific_name / _struct_ref.db_code / _struct_ref.pdbx_db_accession / _struct_ref.pdbx_seq_one_letter_code / _struct_ref_seq.pdbx_db_accession Revision 2.0 Jul 29, 2020 Group Advisory / Atomic model ... Advisory / Atomic model / Data collection / Derived calculations / Structure summary Category atom_site / chem_comp ... atom_site / chem_comp / entity / pdbx_branch_scheme / pdbx_chem_comp_identifier / pdbx_entity_branch / pdbx_entity_branch_descriptor / pdbx_entity_branch_link / pdbx_entity_branch_list / pdbx_entity_nonpoly / pdbx_nonpoly_scheme / pdbx_struct_assembly_gen / pdbx_unobs_or_zero_occ_atoms / pdbx_validate_close_contact / struct_asym / struct_conn / struct_site / struct_site_gen Item _atom_site.B_iso_or_equiv / _atom_site.Cartn_x ... _atom_site.B_iso_or_equiv / _atom_site.Cartn_x / _atom_site.Cartn_y / _atom_site.Cartn_z / _atom_site.auth_asym_id / _atom_site.auth_atom_id / _atom_site.auth_comp_id / _atom_site.auth_seq_id / _atom_site.label_asym_id / _atom_site.label_atom_id / _atom_site.label_comp_id / _atom_site.label_entity_id / _atom_site.type_symbol / _chem_comp.name / _chem_comp.type / _pdbx_struct_assembly_gen.asym_id_list / _pdbx_unobs_or_zero_occ_atoms.auth_asym_id / _pdbx_unobs_or_zero_occ_atoms.auth_atom_id / _pdbx_unobs_or_zero_occ_atoms.auth_comp_id / _pdbx_unobs_or_zero_occ_atoms.auth_seq_id / _pdbx_unobs_or_zero_occ_atoms.label_asym_id / _pdbx_unobs_or_zero_occ_atoms.label_atom_id / _pdbx_unobs_or_zero_occ_atoms.label_comp_id / _pdbx_validate_close_contact.auth_asym_id_1 / _pdbx_validate_close_contact.auth_asym_id_2 / _pdbx_validate_close_contact.auth_seq_id_1 / _pdbx_validate_close_contact.auth_seq_id_2 / _struct_conn.pdbx_dist_value / _struct_conn.pdbx_leaving_atom_flag / _struct_conn.ptnr1_auth_asym_id / _struct_conn.ptnr1_auth_comp_id / _struct_conn.ptnr1_auth_seq_id / _struct_conn.ptnr1_label_asym_id / _struct_conn.ptnr1_label_atom_id / _struct_conn.ptnr1_label_comp_id / _struct_conn.ptnr1_label_seq_id / _struct_conn.ptnr2_auth_asym_id / _struct_conn.ptnr2_auth_comp_id / _struct_conn.ptnr2_auth_seq_id / _struct_conn.ptnr2_label_asym_id / _struct_conn.ptnr2_label_atom_id / _struct_conn.ptnr2_label_comp_id Description / Provider / Type Revision 3.0 Dec 25, 2024 Group Advisory / Atomic model ... Advisory / Atomic model / Data collection / Database references / Derived calculations / Structure summary Category atom_site / chem_comp ... atom_site / chem_comp / chem_comp_atom / chem_comp_bond / database_2 / entity / pdbx_entity_nonpoly / pdbx_entry_details / pdbx_nonpoly_scheme / pdbx_unobs_or_zero_occ_atoms / pdbx_validate_close_contact / struct_asym / struct_conn Item _atom_site.B_iso_or_equiv / _atom_site.Cartn_x ... _atom_site.B_iso_or_equiv / _atom_site.Cartn_x / _atom_site.Cartn_y / _atom_site.Cartn_z / _atom_site.auth_asym_id / _atom_site.auth_atom_id / _atom_site.auth_comp_id / _atom_site.auth_seq_id / _atom_site.label_asym_id / _atom_site.label_atom_id / _atom_site.label_comp_id / _atom_site.label_entity_id / _atom_site.type_symbol / _chem_comp.name / _chem_comp.pdbx_synonyms / _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _entity.formula_weight / _entity.pdbx_description / _entity.src_method / _pdbx_entity_nonpoly.comp_id / _pdbx_entity_nonpoly.name / _pdbx_nonpoly_scheme.auth_mon_id / _pdbx_nonpoly_scheme.auth_seq_num / _pdbx_nonpoly_scheme.entity_id / _pdbx_nonpoly_scheme.mon_id / _pdbx_nonpoly_scheme.pdb_mon_id / _pdbx_nonpoly_scheme.pdb_seq_num / _pdbx_nonpoly_scheme.pdb_strand_id / _struct_asym.entity_id
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords Function and homology information
Function and homology information
 X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.908 Å
MOLECULAR REPLACEMENT / Resolution: 2.908 Å  Authors
Authors Citation
Citation Journal: Nature / Year: 2018
Journal: Nature / Year: 2018 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 6bpl.cif.gz
6bpl.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb6bpl.ent.gz
pdb6bpl.ent.gz PDB format
PDB format 6bpl.json.gz
6bpl.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/bp/6bpl
https://data.pdbj.org/pub/pdb/validation_reports/bp/6bpl ftp://data.pdbj.org/pub/pdb/validation_reports/bp/6bpl
ftp://data.pdbj.org/pub/pdb/validation_reports/bp/6bpl Links
Links Assembly
Assembly
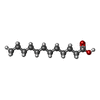
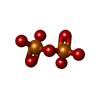
 Components
Components













 X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation SYNCHROTRON / Site:
SYNCHROTRON / Site:  SSRL
SSRL  / Beamline: BL12-2 / Wavelength: 1 Å
/ Beamline: BL12-2 / Wavelength: 1 Å Processing
Processing MOLECULAR REPLACEMENT / Resolution: 2.908→39.9 Å / SU ML: 0.46 / Cross valid method: FREE R-VALUE / σ(F): 1.37 / Phase error: 31.42
MOLECULAR REPLACEMENT / Resolution: 2.908→39.9 Å / SU ML: 0.46 / Cross valid method: FREE R-VALUE / σ(F): 1.37 / Phase error: 31.42  Movie
Movie Controller
Controller














 PDBj
PDBj













