+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6aw1 | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of CEACAM3 | ||||||
 Components Components | Carcinoembryonic antigen-related cell adhesion molecule 3 | ||||||
 Keywords Keywords | CELL ADHESION | ||||||
| Function / homology |  Function and homology information Function and homology informationregulation of immune system process / specific granule membrane / protein tyrosine kinase binding / Cell surface interactions at the vascular wall / Neutrophil degranulation / cell surface / signal transduction / plasma membrane Similarity search - Function | ||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.1 Å MOLECULAR REPLACEMENT / Resolution: 2.1 Å | ||||||
 Authors Authors | Bonsor, D.A. / Sundberg, E.J. | ||||||
 Citation Citation |  Journal: EMBO J. / Year: 2018 Journal: EMBO J. / Year: 2018Title: TheHelicobacter pyloriadhesin protein HopQ exploits the dimer interface of human CEACAMs to facilitate translocation of the oncoprotein CagA. Authors: Bonsor, D.A. / Zhao, Q. / Schmidinger, B. / Weiss, E. / Wang, J. / Deredge, D. / Beadenkopf, R. / Dow, B. / Fischer, W. / Beckett, D. / Wintrode, P.L. / Haas, R. / Sundberg, E.J. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6aw1.cif.gz 6aw1.cif.gz | 103.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6aw1.ent.gz pdb6aw1.ent.gz | 78.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6aw1.json.gz 6aw1.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/aw/6aw1 https://data.pdbj.org/pub/pdb/validation_reports/aw/6aw1 ftp://data.pdbj.org/pub/pdb/validation_reports/aw/6aw1 ftp://data.pdbj.org/pub/pdb/validation_reports/aw/6aw1 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6avzC  6aw0C  6aw2C  6aw3C  2qsqS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 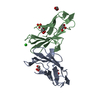
| ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 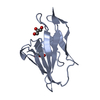
| ||||||||||||||||||
| 2 | 
| ||||||||||||||||||
| 3 | 
| ||||||||||||||||||
| Unit cell |
| ||||||||||||||||||
| Components on special symmetry positions |
| ||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: _ / Ens-ID: 1 / Beg auth comp-ID: ALA / Beg label comp-ID: ALA / End auth comp-ID: TYR / End label comp-ID: TYR / Refine code: _ / Auth seq-ID: 0 - 107 / Label seq-ID: 2 - 109
|
- Components
Components
-Protein , 1 types, 2 molecules AB
| #1: Protein | Mass: 12045.568 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)  Homo sapiens (human) / Gene: CEACAM3, CD66D, CGM1 / Production host: Homo sapiens (human) / Gene: CEACAM3, CD66D, CGM1 / Production host:  |
|---|
-Non-polymers , 5 types, 73 molecules 








| #2: Chemical | ChemComp-GOL / #3: Chemical | ChemComp-CL / | #4: Chemical | ChemComp-SO4 / | #5: Chemical | ChemComp-PEG / | #6: Water | ChemComp-HOH / | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.56 Å3/Da / Density % sol: 65.41 % |
|---|---|
| Crystal grow | Temperature: 298 K / Method: vapor diffusion, hanging drop / pH: 5.5 Details: 1.0M Ammonium Sulphate, 1% PEG 3350, 0.1M Bis Tris pH 5.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRL SSRL  / Beamline: BL12-2 / Wavelength: 0.9795 Å / Beamline: BL12-2 / Wavelength: 0.9795 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Jan 21, 2015 / Details: Flat Si Rh coated M0 |
| Radiation | Monochromator: Si(111) / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength: 0.9795 Å / Relative weight: 1 |
| Reflection | Resolution: 2.1→80.46 Å / Num. obs: 20361 / % possible obs: 98.9 % / Redundancy: 5 % / Rmerge(I) obs: 0.057 / Rpim(I) all: 0.041 / Net I/σ(I): 16.7 |
| Reflection shell | Resolution: 2.1→2.16 Å / Redundancy: 5.1 % / Rmerge(I) obs: 1.022 / Mean I/σ(I) obs: 1.8 / Num. unique all: 1645 / Rpim(I) all: 0.748 / % possible all: 99.3 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure:  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 2QSQ Resolution: 2.1→80.46 Å / Cor.coef. Fo:Fc: 0.964 / Cor.coef. Fo:Fc free: 0.96 / SU B: 12.373 / SU ML: 0.149 / Cross valid method: THROUGHOUT / ESU R: 0.169 / ESU R Free: 0.146 / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.7 Å / Shrinkage radii: 0.7 Å / VDW probe radii: 1.1 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 54.236 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 2.1→80.46 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj


